Figure 3. Structure of ecFabH-CoA Superimposed with ecFabF.
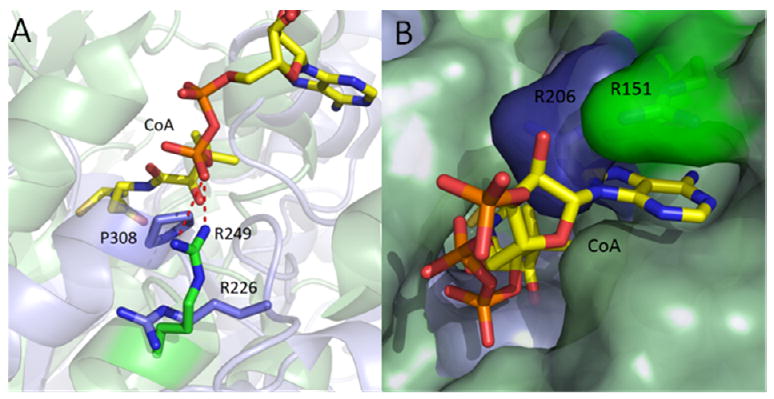
The interaction of CoA with ecFabF was explored by superimposing the active sites and malonyl binding channels of each enzyme with the respective portions of ecFabH complexed with CoA (47, 52). (a) While R249 in ecFabH (green) forms an interaction to the pyrophosphate of the PPant chain, R226 in ecFabF (blue) is flipped 90 degrees and replaced by P308, preventing bond formation to the pyrophophate of CoA. (b) R151 in ecFabH which binds to the adenine portion of CoA is replaced by R206 in ecFabF which apparently cannot form the same favorable interaction. The figure was made using PyMol (52) and the PDB entries 2eft (ecFabH), 2gfw (ecFabF).
