TABLE.
Summarized Index Showing Representative Icons and Detailed Descriptions for Specific Molecules and Features Depicted in Figs. 3–7
| Molecule, Hormone, Matrix-protein, Enzyme, Protein, and Description | Normal | Cleaved | Mutated | Reduced Expression |
|---|---|---|---|---|
| FGF23: Full-length FGF23 is the active form (inhibits phosphate uptake and mineralization in vivo). Cleaved FGF23 is inactive, and a COOH terminal mutation(s) in ADHR is proposed to increase resistance to proteolysis and thus full-length protein stability (activating mutation). FGF23 may also play a role in OHO and HYP. FGF23 cleavage by PHEX is equivocal, although processing may be influenced indirectly by PHEX. |  |
 |
 |
NA |
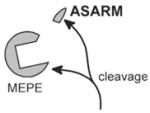
| MEPE: Cleavage of MEPE results in release of ASARM peptide. The ASARM peptide is resistant to proteolysis (most known proteases) and is very stable. MEPE expression is elevated in HYP and is secreted by OHO tumors. Moreover, ASARM peptide inhibits mineralization. In contrast, MEPE ASARM peptide, when part of a matrix-protein (uncleaved from MEPE-protein) and bound to extracellular matrix, may well be required as a nucleator/enhancer of mineralization. Elevated MEPE expression and protease activity in HYP are predicted to result in increased levels of free ASARM peptide. Moreover, free ASARM peptide may also contribute to the inhibition of renal phosphate-uptake (see below). |  |
 |
NA | NA |
| Matrix-proteins required for mineralization (osteocalcin, bone sialoprotein, vitronectin, DMP-1): These proteins are down-regulated in HYP, and the DMP-1 null-mutant has a rickets-like bone phenotype. Of relevance to the functional role of the ASARM peptide present in DMP-1, MEPE, BSP, and OPN is the finding that mineralization enhancement occurs when matrix proteins such as DMP-1 and DSP are irreversibly bound to matrix (collagen). In contrast, unbound matrix phosphoproteins inhibit mineralization. This may well be analogous to the presence of free, protease-resistant, acidic phosphorylated ASARM peptide in Hyp (minhibin osteoblastic-phosphatonin). |  |
NA | NA | 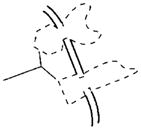 |
| PHEX: This gene is mutated in Hyp, and evidence suggests that one of its functions is to protect MEPE from proteolytic cleavage and thus release of ASARM peptide. PHEX may also indirectly influence FGF23 processing at an extra-osseous site. Although low-level expression of FGF23 does occur in bone, there are no reports of FGF23 expression in primary osteoblasts. However, low-level FGF23 expression has been reported in an SV40 transgenic (stably transformed) normal mouse osteoblastic cell-line (NORM-SV40). Moreover, FGF23 expression in an Hyp SV40 transgenic Hyp-mouse osteoblastic cell-line (Hyp-SV40) was relatively increased. Also, unlike MEPE, there is no correlation with osteoblast developmental expression and FGF23 expression. Direct PHEX cleavage of FGF23 is equivocal (see text for further discussion). | 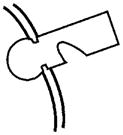 |
NA | 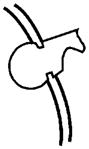 |
NA |
| ASARM peptide (MEPE): In free form, this peptide inhibits mineralization. As part of a matrix protein (MEPE, DMP-1) bound to extracellular matrix, it may well be required as a nucleator/enhancer of mineralization. The ASARM peptide is resistant to most known proteases and is very stable. Elevated MEPE expression and protease activity in HYP are predicted to result in increased levels of ASARM peptide. Moreover, evidence that PHEX protects MEPE from proteolysis suggests that this may also contribute to the increased levels of ASARM peptide in HYP (defective PHEX). Free ASARM peptide may also contribute to the inhibition of renal-phosphate uptake. The renal-phosphate inhibition is likely steric and would exacerbate transcriptional effects on NPT2 expression mediated directly or indirectly by other factors (FGF23). |  |
NA | NA | NA |
| Proteases/Cathepsin B: Protease ECEL-1/DINE, NEP, and cathepsin D are markedly elevated in HYP osteoblasts and bone marrow stem cells. In combination with the documented increased in MEPE expression, this may contribute to a marked elevation of free MEPE ASARM peptide in Hyp. The ASARM peptide is resistant to proteolysis. |  |
NA | NA | NA |
