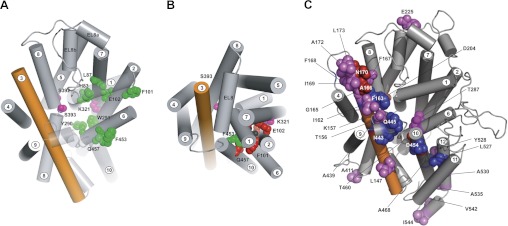
Fig. 8.
A: accessibility of external MTS reagents to hSGLT1 mutants in outward-facing conformation [C2Na2]. The model was generated by threading the human sequence on a model of the inward-facing conformation of vSGLT, made by comparison with the outward-facing conformation of Mhp1 and using the sequence alignment shown in Fig. 2. The model is shown from the external side of the membrane. The residues highlighted in green are cysteine mutants of the gate and ligand binding residues (Fig. 3) that were accessible to polar MTS reagents in the external solution in the [C2Na2] conformation. The mutants of the Na+ site residues S392C and S393C (red) were slightly accessible to methyl methanethiosulfonate (MeMTS). The access of K321C (pink) could not be determined due to low expression. Helices 1 and 10 are transparent for clarity. Note that the TMs are numbered according to the LeuT convention (2). B: accessibility of external MTS reagents to hSGLT1 mutants in inward-facing conformation [C4Na2S]. Accessibility to the residues indicated in A was changed in the inward occluded conformation. Only F453C (green) was accessible to polar cysteine reagents, whereas residues N78C, H83C, L87C, F101C, E102C, Y290, W291, and Q457 (red) were not accessible. S392C and S393C were only accessible (poorly) to MeMTS, and this was independent of conformation. The transparency of helices 1 and 10 (A) has been removed to show the steric hindrance created by these structures. The view of the protein model (Fig. 3) is shown from the external face of the membrane. C: summary of other SGLT1 mutations. Shown is the location of previously reported SGLT1 cysteine mutations that 1) do not significantly alter transport kinetics (violet and blue) or 2) are inhibited (or accessible) to external polar MTS reagents (blue). Those highlighted in red show partial sensitivity to MTS. (See Refs. 3, 12, 14, 15, 19–21, 30, 31, 34, 35, 39; Sala-Rabanal M, Martin M, and Wright EM, unpublished observations; and Jiang X, Hirayama BA, Loo DDF, and Wright EM, unpublished observations.)