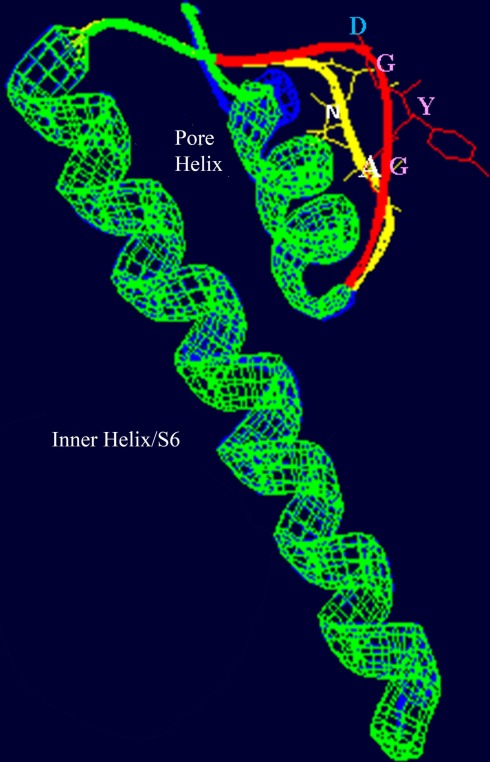
Figure 3.
Superimposed images (redrawn from Leng et al., 2002) of the S6 helix and pore domains of the plant (A. thaliana) CNGC2 sequence and the bacterial (Streptomyces lividans) K+-selective channel KcsA (Doyle et al., 1998; PDB record 1BL8A). Threading of the A. thaliana CNGC2 S6 transmembrane helix and pore region through the KcsA structure identifies the residues “AND” as in the ion conducting pathway where the residues “GYG” (shown in pink) that form the ion selectivity filter of a K+-selective channel are positioned. This suggests that plant CNGCs may have a mechanism for cation conductance that is conserved in P-loop channels. In plants, four separate CNGC peptides are presumed to form a tetrameric structure that shares the “inverted teepee” quaternary structure of animal P-loop channels.