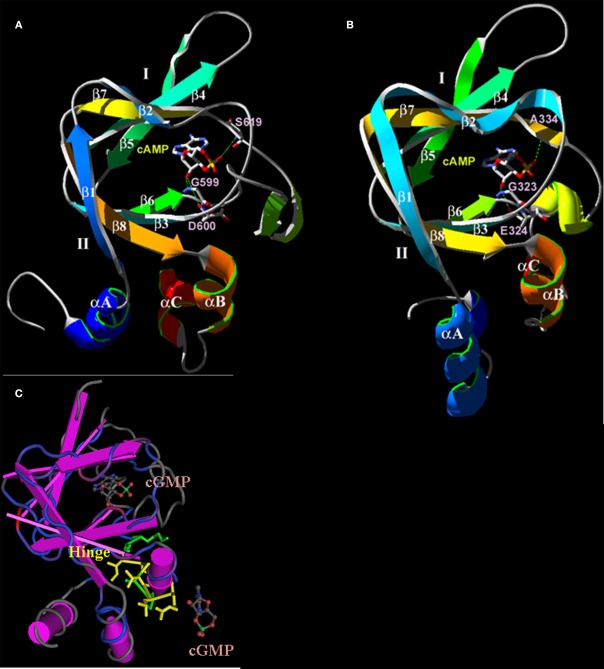
Figure 4.
Predicted three-dimensional structures of the plant CNGC CNBD. (A) A proposed three-dimensional structure of the A. thaliana CNGC2 CNBD generated by threading the sequence through the structure of the cAMP-binding domain of PDB record 1RGS, bovine cAMP-dependent protein kinase A (RIα; Su et al., 1995). (B) Three-dimensional structure of the CNBD of RIα that was used to create the model of the CNGC2 CNBD. The CNBD structures of CNGC2 and RIα share an N-terminal α-helix (αA) and a β-barrel (depicted as ribbons with arrows denoting antiparallel strands) that together bind the cNMP ligand. Republished from Hua et al. (2003a). (C) The tertiary structure of the A. thaliana CNGC6 CNBD (Chikayama et al., 2004; PDB record 1WGP). Amino acid residues comprising the hinge domain are highlighted in yellow; α-helices are depicted as purple cylinders. The β-barrel is shown as straight antiparallel purple ribbons. Two cGMP molecules are shown with the structure, one in the pocket formed between an αA helix and the β-barrel, and a second cGMP molecule (not bound) is depicted at the α-C helix adjacent to the hinge domain.