Abstract
The concentration of mitochondrial oxidative phosphorylation complexes (MOPCs) is tuned to the maximum energy conversion requirements of a given tissue; however, whether the activity of MOPCs is altered in response to acute changes in energy conversion demand is unclear. We hypothesized that MOPCs activity is modulated by tissue metabolic stress to maintain the energy-metabolism homeostasis. Metabolic stress was defined as the observed energy conversion rate/maximum energy conversion rate. The maximum energy conversion rate was assumed to be proportional to the concentration of MOPCs, as determined with optical spectroscopy, gel electrophoresis, and mass spectrometry. The resting metabolic stress of the heart and liver across the range of resting metabolic rates within an allometric series (mouse, rabbit, and pig) was determined from MPOCs content and literature respiratory values. The metabolic stress of the liver was high and nearly constant across the allometric series due to the proportional increase in MOPCs content with resting metabolic rate. In contrast, the MOPCs content of the heart was essentially constant in the allometric series, resulting in an increasing metabolic stress with decreasing animal size. The MOPCs activity was determined in native gels, with an emphasis on Complex V. Extracted MOPCs enzyme activity was proportional to resting metabolic stress across tissues and species. Complex V activity was also shown to be acutely modulated by changes in metabolic stress in the heart, in vivo and in vitro. The modulation of extracted MOPCs activity suggests that persistent posttranslational modifications (PTMs) alter MOPCs activity both chronically and acutely, specifically in the heart. Protein phosphorylation of Complex V was correlated with activity inhibition under several conditions, suggesting that protein phosphorylation may contribute to activity modulation with energy metabolic stress. These data are consistent with the notion that metabolic stress modulates MOPCs activity in the heart.
Keywords: mitochondria, cytochrome oxidase, Complex V, F1-F0ATPase, oxygen consumption, energy metabolism, heart, liver, murine, porcine, rabbit, native gel electrophoresis, mass spectrometry, posttranslational modifications, phosphorylation
mitochondria are dynamic intracellular organelles that play a major role in eukaryotic cellular energy conversion, intermediary metabolism, calcium homeostasis, cell signaling, and apoptosis. With regard to energy conversion, mitochondria are capable of precisely orchestrating the generation of ATP to match the ATP hydrolysis required to perform cellular work. In recent years an increasing body of evidence has revealed that the expression level of mitochondrial proteins is finely tuned to the functional requirements of different tissues and disease states (11, 27, 28, 38–40, 49, 60, 63, 72, 89). Notably, these differences in “protein programming” are realized by regulating most, if not all, of the enzymes in a given metabolic pathway, as opposed to individual rate-limited steps (38). Consistent with this notion, the mitochondrial oxidative phosphorylation complexes (MOPCs) responsible for the generation of ATP are upregulated in tissues with high-energy demands, such as the heart compared with liver (11, 38). Thus adjusting the expression level of MOPCs across different tissues provides a long-term regulatory mechanism for matching maximum mitochondrial ATP production with the maximum work requirements of a given tissue (11, 40, 78). This principle of matching the expression level of metabolic enzymes with the maximum demands of a tissue was first outlined by Weibel and coworkers (87) and termed symmorphosis. While the relative concentration of MOPCs has been shown to be proportional to the maximum metabolic rate across tissues, the mechanisms involved in altering the activity of MOPCs to dynamically modulate ATP production in concert with ATP hydrolysis remains elusive.
The regulation of MOPCs activity can be accomplished by several means. The simplest mechanism involves the feedback of substrates for an enzymatic reaction, such as ADP and Pi, for the ATP synthetic reaction. However, it has been shown in several systems, most notably the heart (see reviews Refs. 6 and 7) and to a lesser extent skeletal muscle (33), that large changes in mitochondrial synthetic rate are not associated with significant alterations in the substrates for ATP production or the net free energy in cytosolic ATP (ΔGATPc). This ability to maintain a near-constant ΔGATPc with changes in workload has been termed metabolic homeostasis (5, 33). Thus simple substrate feedback does not seem adequate to coordinate mitochondrial ATP synthesis with work requirements.
Another mechanism for modulating enzyme function involves covalent posttranslational modifications (PTMs), which can alter activity with little or no change in reaction substrates. Given the observed metabolic homeostasis that occurs during alterations in workload, PTMs are the best candidate for acutely regulating MOPC enzyme activity to meet increases in ATP demand. The classic example of this type of regulation is mitochondrial pyruvate dehydrogenase (PDH), which is inactivated by phosphorylation [i.e., effectively reduces the maximum velocity (Vmax) of the reaction] during times of low activity (18, 46, 70). Ca2+ regulates PDH phosphorylation through modulation of PDH phosphatase I (24, 65). In addition, Ca2+ also alters the affinity of isocitrate dehydrogenase (ICDH) and α-ketoglutarate dehydrogenase (αKDH) for carbon substrates through noncovalent allosteric interactions (20).
The question remains whether PTMs could play a wider role in the acute regulation of mitochondrial energy conversion. Recent studies have revealed numerous persistent PTMs on MOPCs, including acetylation (2, 10), phosphorylation (4, 37, 52, 77), nitrosylation (82), ADP-ribosylation (50), and oxidation (53). Additionally, several PTMs have been correlated with alteration in Complex I (17, 64, 79), Complex IV (1, 12, 52), and Complex V (76, 84) activities. This list of mitochondrial protein PTMs is growing rapidly with the development of sophisticated mass spectrometry methodologies; however, very few functional consequences of these interactions have been established. Thus, whereas a wide variety of PTMs are present in the MOPCs, the functional significance of these sites is poorly defined.
In the current study, we hypothesize that PTMs modulate the activity of MOPCs to allow a tissue to maintain a metabolic homeostasis in the face of persistent and acute changes in energy demand. This hypothesis addresses the metabolic activity “downstream” of the citric acid cycle reactions discussed above. We proposed that this coordinated system of PTMs would be more prevalent in tissues and animals with large dynamic ranges in ATP generation compared with those systems operating near their maximum capacity. Consistent with these regulators being reversible PTMs, we reasoned that they would persist in isolated MOPC activity assays, as was originally demonstrated for PDH (22). To test this, we screened for regulatory PTMs using the activity of extracted MOPCs. We defined MOPC activity as the catalytic turnover rate per mole of complex. To estimate energy demand, we calculated metabolic stress [i.e., energy conversion rate/maximum energy conversion rate (29)] where the maximum energy conversion rate was assumed to be proportional to total MOPC content.
We evaluated our hypothesis using three systems. First, we focused on “resting” conditions in the pig heart and liver. These tissues were selected because their MOPCs have only minor differences in protein sequence but possess very different dynamic ranges in ATP production. For instance, the large animal heart has a dynamic range of ATP production that exceeds 10-fold (59, 86), whereas the liver has a dynamic range of less than 2-fold (45, 54, 80). Second, MOPC activity was determined in an allometric series (i.e., pig, rabbit, and mouse) to take advantage of the heart's decreasing resting metabolic rate and increasing dynamic range of energy metabolism with increasing body size. Third, we acutely altered the workload of the pig heart in vivo and the rabbit heart in vitro to determine whether the persistent inhibition of MOPC activity in the “resting” heart could be removed by an increase in workload.
In this study we demonstrate that MOPC activity parallels metabolic stress. We show that MOPC activity is persistently inhibited at “rest” in animals and tissues with large dynamic ranges of ATP production, with the ability to release this inhibition during increased workload. Finally, the in vitro activity of Complex V was inversely correlated with γ-subunit protein phosphorylation consistent with a role of protein phosphorylation in the acute modulation of Complex V activity and potentially the maintenance of the metabolic homeostasis in heart.
MATERIALS AND METHODS
Materials.
Salts and Pi were purchased from Sigma (St. Louis, MO). Two-dimensional (2D) gel electrophoresis reagents CyDyes, equipment, and software were purchased from GE Healthcare (Piscataway, NJ). Native PAGE buffers, reagents, and gels were purchased from Invitrogen (Carlsbad, CA). 32P (10 mCi/ml) was purchased from PerkinElmer (Boston, MA).
Mitochondrial isolation.
All procedures performed were in accordance with the Animal Care and Welfare Act (7 U.S.C. 2142 § 13) and approved by the NHLBI ACUC. Heart mitochondria were isolated from animals using an in situ cold perfusion to prevent warm ischemia and remove blood and extracellular Ca2+ as described (30). One modification was that 1 mM K2PO4 was added to buffer A (0.28 M sucrose, 10 mM HEPES, 1 mM EDTA, and 1 mM EGTA; pH 7.1) for the first mitochondrial resuspension. This was done to avoid phosphate depletion of the mitochondria matrix, as described previously (4). Mitochondria were then washed twice with buffer A alone, once with buffer B (137 mM KCl, 10 mM HEPES, 2.5 mM MgCl2, and 0.5 mM K2EDTA), and finally resuspended in buffer B. Liver mitochondria were isolated from the same animals as the heart mitochondria following a similar protocol (4). After euthanasia the liver was immediately removed and flushed with 1 liter of cold buffer A to chill the tissue and remove blood. Only visually blanched tissue was used for isolation. After fat and connective tissue were removed, the liver was finely chopped in cold buffer A, homogenized twice with a loose-fitting tissue grinder, and centrifuged at 600 g for 10 min at 4°C. The mitochondrial pellet was washed and resuspended following the same protocol described above for the heart.
Mitochondrial preparations were tested for viability by measuring the respiratory control ratio (RCR), which involved taking the ratio of the rate of oxygen consumption in the presence and absence of ADP (1 mM) at 37°C with the following incubation medium: buffer B containing 5 mM potassium-glutamate, 5 mM potassium-malate, and 1 mM Pi. To accept a mitochondrial preparation, the RCR had to exceed 8 in the heart and 5 in the liver to assure a well-coupled system. The reasons for the lower RCR in liver are unknown.
In this study, isolated mitochondria were used for protein analysis only. Viability was evaluated to assure complexes isolated were from functional mitochondria. No attempt was made to correlate in vitro mitochondria activity using State 3/4 respiration between organs or animals. The reasons for avoiding this type of comparison included the following: 1) Different carbon substrate oxidation capacities in different tissues (38). Thus direct comparisons are nearly impossible to make with a given reduced carbon source. 2) Alterations in the matrix milieu induced by the isolation process, such as Pi, Ca2+, and volume, that could alter the distribution of PTMs from the in vivo state. The alteration in PTM with isolation was demonstrated in some of the original work on PDH (43) that showed a time-dependent alteration of PDH activity in isolated mitochondria. We have also found that isolated mitochondria complex activities are generally higher than assays performed on complexes rapidly isolated directly from tissue biopsies consistent with the isolation environments influencing the PTM profile of these preparations.
Spectrophotometric cytochrome quantitation.
Mitochondrial cytochrome a,a3 (cyto a) content was assayed from an oxidized-reduced spectrum of a Triton X-100-treated mitochondrial suspension as previously described (9). Briefly, Triton X-100-treated extracts were assayed at 605 nm (with reference created from a linear regression between 575 and 630 nm) using cyanide to generate a fully reduced spectrum in the presence of blood and myoglobin. The cyto a content was determined using an extinction coefficient of 10.8 mM−1·cm−1 with cyanide as a reducing agent and 12 mM−1·cm−1 when sodium hydrosulfite was used in mitochondria extracts. Because of the interferences from hemoglobin and myoglobin, cyto c and b were not determined in whole tissue extracts using this approach.
The cyto a measurements were used to determine the content of oxidative phosphorylation enzymes in different tissues and species. Cyto a measurements were reported as 10−9 moles (nmole) of cyto a per mg mitochondrial protein or gram of wet heart weight. Since the mitochondria of the heart and liver vary considerably in their composition (i.e., heart mitochondria per milligram of mitochondrial protein contain more oxidative phosphorylation enzymes, whereas liver mitochondria contain more urea cycle enzymes), we used cyto a content to match oxidative phosphorylation enzyme content between tissues. Native in-gel activity assays were loaded per cyto a content and further normalized per mole of MOPC based on Coomassie blue staining (see Complex activity measurements).
32P-labeling of intact mitochondria.
32P-labeling of intact mitochondria and isolated Complex V was performed as previously described (4). Briefly, intact pig heart and liver mitochondria were incubated at 37°C for 20 min in oxygenated Buffer C (125 mM KCl, 15 mM NaCl, 20 mM HEPES, 1 mM EGTA, 1 mM EDTA, 5 mM MgCl2, 5 mM potassium-glutamate, and 5 mM potassium-malate; pH 7.1) and 250 μCi/mg of 32P. Complex V was then isolated using an immunocapture kit (Mitosciences, Eugene, OR), according to the manufacturer's instructions. Forty micrograms of purified 32P-labeled Complex V from each tissue were then analyzed by 2D gel electrophoresis as described below.
2D gel electrophoresis and analysis.
32P-labeled Complex V and CyDye-labeled heart and liver mitochondrial proteins were analyzed by 2D gel electrophoresis, as previously described (4). Proteins were separated first by charge via 24-cm IPG strips (pH 3–10 NL) and then by mass on 10–15% gradient gels. 32P-labeled protein gels were stained with Coomassie blue, dried, exposed to a phosphorscreen, and imaged; CyDye-labeled gels were imaged immediately following electrophoresis, as described previously (4). To identify proteins from the 2D gels, paired nonradiolabeled gels were picked using the Ettan Spot Handling Workstation (GE Healthcare) and identified using a MALDI-TOF/TOF instrument (4700 Proteomics Analyzer, Applied Biosystems), as previously described (4).
The differential in-gel electrophoresis (2D-DIGE) was also used to estimate absolute protein concentrations in the different tissues, using the relative areas of the peaks and the molecular weights versus proteins of known concentration. For these studies we used the absolute concentration of Complex IV (cyto a), determined spectrophotometrically, as a reference for quantization of other proteins in the 2D-DIGE gel.
Isobaric tags for relative and absolute quantitation processing.
To obtain more comprehensive protein coverage and relative protein concentrations as well as information on subunit composition, a mass spectrometry study using isobaric tags for relative and absolute quantitation (iTRAQ)-labeling was performed on heart and liver mitochondria from the pig and mouse. The iTRAQ approach overcomes the quantitative limitation of label-free techniques (39). One hundred micrograms of acetone-precipitated protein lysate was iTRAQ-labeled according to the manufacturer's instructions (Invitrogen). Isobaric tagging was performed from mass-to-charge (m/z) 113–116 for the mouse heart samples and m/z 117–121 for the mouse liver samples. The pig heart samples were labeled with tags m/z 114–116 and liver samples with m/z 118–121. All iTRAQ data for mouse and pig data sets were presented as the ratio of heart proteins to liver proteins. Both pig and mouse sets were prepared separately but followed the same methods throughout. After quenching the labeling reaction, the resulting peptide mixtures were combined and dried until the final volume of 100 μl was achieved. The combined peptide digest was resuspended in 900 μl of 0.1% formic acid and desalted using Waters Oasis HLB 1-cm3 cartridges (Milford, MA) per the manufacturer's instructions using acetonitrile instead of methanol. Eluent was dried and resuspended with 100 μl strong cation exchange chromatography buffer A (10 mM KH2PO4/25% acetonitrile, pH 3.0).
Strong cation exchange chromatography.
An Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA) was used to separate the peptides by their charge using a polysulfoethyl A column; 200 × 2.1 mm, particle size 5 μm, 200 Å (Poly LC, Columbia, MD). A 60-min linear ramp from 0% to 40% buffer B (10 mM KH2PO4-500 mM KCl-25% ACN, pH 2.7) was used to separate the peptides. Column temperature was maintained at room temperature, and a flow rate of 200 μl/min was maintained throughout the run. Fractions were collected at 1-min intervals on a 96-well microtiter plate for a total of 60 fractions. The chromatographic peaks were monitored using the built in UV detector (214 nm), and fractions were combined for a final count of 23 fractions. Each fraction was desalted using Waters Oasis HLB 1 cm3 cartridges (Milford, MA) per the manufacturer's instructions using acetonitrile instead of methanol.
Mass spectrometry LC MS/MS: orbitrap velos.
Liquid chromatography tandem mass spectrometry was performed using an Eksigent nanoLC-Ultra 1D plus system (Dublin, CA) coupled to an LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific, San Jose, CA). The peptide digest of 10 μl was first loaded onto an Zorbax 300SB-C18 trap column (Agilent, Palo Alto, CA) at a flow rate of 6 μl/min for 6 min and then separated on a reversed-phase PicoFrit analytical column (New Objective, Woburn, MA) using a 40-min linear gradient of 5–40% acetonitrile in 0.1% formic acid at a flow rate of 250 nl/min. LTQ-Orbitrap Velos settings were as follows: spray voltage 1.5 kV; full MS mass range: m/z 300 to 2,000, operated in positive ion mode with data-dependent acquisition. A single full-scan MS in the Orbitrap (30,000 resolution, 300–2,000 m/z) was followed by six data-dependent MS2 scans for precursor ions above a threshold ion count of 10,000, using the HCD cell with the resolution set to 7,500 and 45% normal collision energy.
Mass spectrometry data analysis.
The raw files generated were analyzed for protein identification and quantification using Proteome Discoverer v1.2 software (Thermo Fisher Scientific, San Jose, CA) using Mascot search engine v.2.3 (http://biospec.nih.gov) managed and weekly updated protein databases by the Center for Information Technology at the NIH. The MS/MS spectra were searched against the Swiss-Prot Protein Knowledgebase (Sprot) combined pig, bovine, and human database (release 2011_03). The combined pig, bovine, and human database was used because the pig protein database is partially annotated. For the mouse samples, the taxonomy was set to house mouse. The enzyme parameters were limited to trypsin with a maximum miscleavages set to 2. The selection criteria were the following: variable modifications; oxidation (M), deamidation (NQ), iTRAQ 8plex (Y); fixed modifications, (MMTS) methyl methanethiosulfonate (C), NH2-terminal iTRAQ 8plex, iTRAQ8plex (K); 10 ppm for precursor ions and 0.05 Da for fragment ions. The quantitative measurements were performed in the MS/MS scan using the iTRAQ reporter ions. Software normalization was not performed. The automatic decoy database search option was selected on Mascot, and the high confidence peptides were only used for protein identification that contained a false discovery rate (FDR) <1% at the peptide level. Briefly, every time a peptide sequence search is performed on a target database, a random sequence of equal length is automatically generated and tested. The statistics for matches are calculated and a peptide significance is generated, and an in-depth explanation can be found www.matrixscience.com. Protein identifications with two or more unique peptides were selected for quantitative analysis. All mass spectra used in this study are publicly available at the Proteome Commons Tranche web site (https://proteomecommons.org/group.jsp?i=219/).
Complex activity measurements.
The activities of MOPCs were determined with various assays within Blue Native polyacrylamide gel electrophoresis (BN-PAGE). BN-PAGE gels were used to isolate and measure Complex I, Complex IV, and Complex V activity in isolated pig heart and liver mitochondria; in vivo biopsy samples from pig heart and liver; and whole tissue extracts from pig, rabbit, and murine heart and liver. Since heart and liver have different amounts of MOPC per milligram of mitochondria, cyto a measurements (obtained spectrophotometrically) were used to normalize MOPC content between tissues. Cyto a (Complex IV) was used as a reference because its ratio to Complexes I and V was constant based on the 2D-DIGE gels. This finding was confirmed by comparing the ratio of Coomassie staining intensity for Complexes I and V to Complex IV in BN-PAGE, which was essentially identical to using cyto a as a reference for the protein loading.
BN-PAGE relies on the binding of Coomassie blue to mitochondrial protein complexes to enhance their differential migration during the PAGE process (74). For isolated mitochondria, BN-PAGE was performed on mitochondrial pellets according to the Invitrogen protocol for the Native PAGE Novex Bis-Tris Gel System, with dodecyl maltoside as the detergent. Since liver contains fewer moles of MOPC per milligram of mitochondrial protein, isolated mitochondrial pellets were solubilized per milligram (to maintain a correct dodecyl-maltoside-to-protein ratio) and then loaded per picomole of cytochrome a (to ensure matched cytochrome content). Four-16% 1-mm bis-Tris gels were used, and 50 pmol of mitochondrial protein were loaded per lane. BN-PAGE was performed at 4°C for 1 h at 150 V and then for 1.3 h at 250 V. Gels used for Complex V activity assays remained in dark cathode buffer for the entire run, whereas gels used for Complex I and Complex IV activity assays switched to light cathode buffer after the first hour of electrophoresis.
After electrophoresis, BN-PAGE gels were photographed to accurately quantify the Coomassie signal (i.e., protein content), and activity assays were then performed. Assays were performed using literature procedures for Complexes I and IV (44) and Complex V (14) with several modifications. For the Complex I activity assay, 0.75 g of nitrotetrazolium blue (NBT) were used and the assay was allowed to proceed for 5 min. For the Complex IV activity assay, 0.01 g of cytochrome c from bovine was used and the assay was allowed to proceed for 10 min. For the Complex V activity assay, the assay buffers were adjusted to a pH of 7.8, and the incubation steps were as follows: preincubation buffer (35 mM Tris·HCl, 270 mM glycine, 14 mM MgSO4, pH 7.8) for 20 min and incubation buffer [35 mM Tris·HCl, 270 mM glycine, 14 mM MgSO4, 0.2% (wt/vol) Pb(NO3)2, 8 mM ATP, pH 7.8] for 20 min. Great care was taken to avoid saturation. This was especially problematic with liver and mouse heart samples, which were relatively the most active. All gels were photographed immediately after completion of the activity assay. Complex I and Complex IV gels were photographed on a light box with no filtering. Complex V gels were placed on a flat flask containing a saturated solution of Coomassie Blue dye; this optimized the contrast of the scattered light from the reaction products. These gels were then photographed using an infrared light and Wratten filter (Kodak, Rochester, NY) to avoid the absorbance by Coomassie. Activities are reported as ratios or relative to the control condition, based on a given gel.
For in vivo biopsy samples or whole tissue homogenates, ∼0.1 g of freshly perfused tissue (trimmed of fat and connective tissue) was added to 1 ml of buffer A, minced into small pieces, and homogenized with a Virtishear for 5 s for heart and 1 s for liver tissue on ice. Samples were then centrifuged at 10,000 g for 5 min at 4°C, supernatants were removed, and pellets were resuspended in 500 ml of buffer B. This step was repeated, and 1 nanomole of cyto a from the suspension was used for BN-PAGE activity assays.
Given differences in the Native PAGE modalities, clear native (CN)-PAGE gels were also run to confirm BN-PAGE results and utilize the oligomycin-sensitivity feature of the CN-PAGE (90). We preferred to use BN-PAGE because protein content and activity could be obtained from the same gel. When measuring activity via CN-PAGE, two gels were required: one gel was used to measure activity and a paired gel was stained with Coomassie blue and used to determine protein concentration. CN-PAGE samples were processed in the same manner as described for BN-PAGE above, except no Coomassie blue additive was included. Instead, an equivalent volume of 4× Native PAGE buffer was used. High-resolution CN-PAGE electrophoresis buffers were chosen according to previous studies (90). To assess Complex I and Complex V activity by CN-PAGE, the electrophoresis buffers used were the following: anode buffer (25 mM imidazole-HCl, pH 7.0) and cathode buffer 1 (50 mM Tricine, 7.5 mM imidazole, 0.02% dodecyl maltoside, 0.05% deoxycholate, pH 7.0). To assess Complex IV activity by CN-PAGE, the buffers used were the following: anode buffer (same as above) and cathode buffer 2 (50 mM Tricine, 7.5 mM imidazole, 0.05% deoxycholate, 0.05% Triton X-100, pH 7.0). CN-PAGE was run on 4–16% bis-Tris gels (Invitrogen), and samples were loaded as described above for BN-PAGE. CN-PAGE electrophoresis was conducted at 4°C at 150 V for 1 h, followed by 250 V for 1.4 h. Activity assays were performed as described for BN-PAGE, with the notable exception that Complex V showed oligomycin sensitivity when assayed with CN-PAGE.
Because of to the importance of these assays in the interpretation of these data, the complete characterization of Native PAGE assays is shown in Fig. 1.
Fig. 1.
Native PAGE activity assay characterization. A: blue native (BN)-PAGE Complex IV activity assay characterization. B: BN-PAGE Complex V activity assay characterization. C: clear native (CN)-PAGE Complex IV activity assay characterization. D: CN-PAGE Complex V activity assay characterization. For A–D: 1) Coomassie-stained native gel; 2) gel activity assay at 10, 20, 40, and 60 min; 3) a plot of activity relative to protein concentration; and 4) a plot of activity as a function of time for the 25-, 50-, and 75-μg lanes. E: CN-PAGE Complex V activity assay characterization with ethanol control (E1)or 50 μM oligomycin (E2). Left, paired Coomassie-stained CN-PAGE gel; right, Complex V activity data at 10, 20, 40, and 60 min.
In vivo biopsy studies in pig hearts.
To obtain biopsy samples, pigs (∼50 kg) were given intramuscular injections (in mg/kg: 25 ketamine, 0.5 midazolam, and 0.01 glycopyrrolate) for sedation. This was followed by intubation with an 8-mm endotracheal tube and anesthesia with 2% Isoflurane and a mixture of oxygen and medical air 35%:65% (Siemen's Kion Ventilator/Anesthesia machine). Blood gases, heart rate, and arterial blood pressure were continuously monitored. A midline sternotomy was performed to expose the tissues. The heart was supported by a pericardial cradle, directly exposing it for biopsy collection. Biopsies were collected from the heart by angling a tissue core biopsy system with a 12-gauge coaxial biopsy needle (Bard Magnum Biopsy Systems, Tempe, AZ) along the long axis of the heart and penetrating roughly halfway through the ventricle wall. Liver biopsy samples were collected from the same lobe. Three biopsy samples (∼0.5 g per biopsy) were obtained per organ, immediately placed in ice-cold buffer A (0.28 M sucrose, 10 mM HEPES, 1 mM EDTA, and 1 mM EGTA; pH 7.1), and processed for BN-PAGE analysis as described above.
In vivo biopsy samples were also obtained from pig hearts under different workload conditions, mediated by dobutamine infusion. Dobutamine is a β sympathetic receptor agonist commonly used to increase cardiac inotropy and to a lesser extent heart rate in anesthetized animals (85). The pig was prepared as described above, except biopsy lacerations were sutured to prevent further bleeding. For each experimental condition, three biopsies (∼0.5 g per biopsy) were taken. The control condition was at rest (predobutamine). Dobutamine was then infused at a dose of 20 μg·kg−1·min−1, and biopsies were taken once a maximum heart rate and pressure were reached. For the current study, “maximum” is defined as the peak heart rates and pressures attained during constant monitoring. To assist with venous return to the heart, the pig's feet were elevated considerably and weights were placed on the abdomen. Dobutamine infusion was then stopped, and 15 min after the pig's heart rate and pressures returned to predobutamine levels, three additional biopsies were taken. All biopsy samples were taken from the same plane of the left ventricle, but to avoid damaged tissue, great care was exercised not to take biopsies from previously sampled regions. Biopsy samples were processed for BN-PAGE analysis as described above. Heart rates and blood pressures were monitored throughout this experiment to calculate the rate pressure product.
The transmural activity of Complex V across the left ventricle wall was determined to establish whether it is homogenous and how critical sampling with a biopsy needle would be with regards to depth. The heart was surgically removed and immediately flushed with ice-cold isolation buffer as described above. The left ventricle was sectioned off from the right ventricle, and a basal section of left ventricle free wall was uniformly cut out. This piece was then cut into sections of inner, mid, and outer weighing ∼0.1 g. The inner, mid, and outer tissue sections were then processed for the CN-PAGE Complex V activity assay, as reported above. A Bradford protein assay was completed to normalize each sample for protein content. No significant differences in Complex V activity was found as a function of transmural position (Fig. 2). Thus differential contributions of transmural tissue to the biopsy samples transmurally would not be a source of variability in Complex V activity.
Fig. 2.
Transmural Complex V activity. In 3 pigs, transmural tissue samples from the outer, middle, and inner regions of the left ventricle were assayed for Complex V activity. A: raw CN-PAGE data; B: quantitative data.
Perfused rabbit heart studies.
To confirm the in vivo dobutamine studies in the pig heart and obtain a larger physiological dynamic range, rabbit hearts were Langendorff-perfused in vitro as previously reported (32). Briefly, hearts were removed from the anesthetized animal and placed in ice-cold saline for transfer to the perfusion apparatus. The hearts were perfused at 37°C at a constant pressure of 100 mm H2O with a coronary flow of ∼50 ml/min. The perfusion medium was composed of (in mM) 115 NaCl, 4 KCl, 1.6 CaC12, 1.4 MgSO4, 1 KHPO4, 25 NaHCO3, 5.6 glucose, and 3 Na l-lactate. The solution was continuously equilibrated with 95% O2 and 5% CO2 to maintain the pH at 7.4. Heart rate, perfusion pressure, and developed pressure were monitored continuously. Three conditions were examined as the following: 1) control perfusion (1.25 h); 2) 1 h control perfusion followed by 0.25 h of dobutamine 3-titration to obtain an approximately threefold increase in heart rate; and) 1.17 h control perfusion, followed by 0.08 h of KCl arrest. For harvesting, the hearts were perfused with ice-cold saline while still on the perfusion apparatus. The left ventricle was then excised and trimmed of fat and connective tissue. Approximately 0.5 g of tissue was homogenized in 5 ml of buffer A, and samples were processed for analysis by Native-PAGE as described above.
RESULTS
Content of mitochondrial oxidative phosphorylation complexes in the heart and liver.
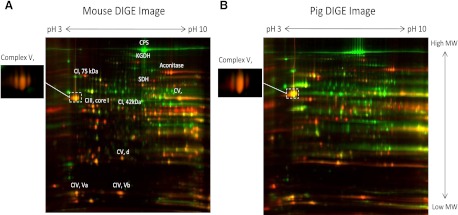
To determine the molar activities of the MOPCs in the heart and liver, the concentration of each Complex had to be obtained. Three methods were utilized to accomplish this task: optical spectroscopy of cytochrome a,a3 (cyto a, which is an element of Complex IV), 2D-DIGE, and iTRAQ mass spectrometry. Since the protein extraction procedures for optical spectroscopy differed significantly from those used for 2D-DIGE and iTRAQ, these measures can be treated independently, free of systematic protein processing errors. We first applied these techniques to isolated heart and liver mitochondria from pig and mouse. With the use of optical spectroscopy, the absolute cyto a content was determined: in pig mitochondria, heart (nmol/mg mitochondrial protein) 0.9 ± 0.1 (n = 4), liver 0.2 ± 0.03 (n = 4); in murine mitochondria, heart 1.0 ± 0.3 (n = 3), liver 0.15 ± 0.01 (n = 6). The concentration of cyto a was significantly higher in the heart in both species, with a heart-to-liver ratio of 4.5 in pig and 6.7 in mouse. These data are consistent with the results of Benard et al. in the rat (11). Additionally, the relative concentration of Complexes I, II, III, IV, and V in heart and liver mitochondria from the pig and mouse were determined with 2D-DIGE and iTRAQ. Consistent with the cyto a measurements and previous studies (11, 27, 38, 40, 60, 63), the 2D-DIGE (Fig. 3, A–B) and iTRAQ (see online supplemental Table S1) studies showed that the concentration of MOPCs is higher in heart than liver mitochondria. The complete iTRAQ datasets for pig (online Supplemental Table S2) and mouse (online Supplemental Table S3) are provided. Table 1 presents a quantitative summary of the heart-to-liver ratios of MOPCs content in pig mitochondria using optical spectroscopy, 2D-DIGE, and iTRAQ. Good agreement was generally found between methodologies, with an exception being the heart-to-liver ratio of Complex I, which was shown to be ∼40% lower with 2D-DIGE. Using 2D-DIGE to quantify relative protein concentrations is complicated by the fact that proteins frequently undergo posttranslational modifications, can be relatively low in abundance, and rarely exist discretely within a gel. The Complex I subunits are especially prone to these complications, which likely accounted for the lower heart-to-liver ratio that was determined with 2D-DIGE. A comparison of the pig and mouse data revealed nearly identical heart-to-liver ratios for MOPC content, with the exception of Complex II (i.e., succinate dehydrogenase and electron transfer flavoprotein), which was relatively lower in mouse (see online Supplemental Tables S2 and S3 and discussion). Thus these studies showed that MOPC content is higher in the heart than the liver and underline the notion that simply using mitochondrial volume to compare the aerobic metabolic capacity between tissues is not reliable.
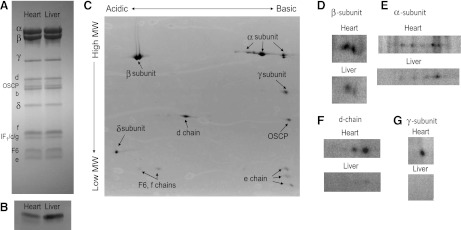
Fig. 3.
Mouse (A) and pig (B) where heart proteins are labeled red and liver green. The β subunit of Complex V is shown as an inset, since its color saturated at the window level necessary to reveal a majority of proteins.
Table 1.
Comparison of mean porcine heart and liver contents using optical spectroscopy, DIGE and iTRAQ
| Mitochondrial Protein | Optical Spectroscopy | 2D-DIGE | iTraQ |
|---|---|---|---|
| Citric acid cycle | ND | 4.1 | 4.1 |
| Complex 1 | ND | 2.2 | 3.7 |
| Complex II | ND | 1.5 | 1.4 |
| Complex III | ND | 4.2 | 4.8 |
| Complex IV | 4.5 | 3.9 | 4.5 |
| Complex V | ND | 3.4 | 3.8 |
The data from two-dimensional differential in-gel electrophoresis (2D-DIGE) and isobaric tags for relative and absolute quantitation (iTRAQ) represent the means of all subunits detected in the respective approach. Complete iTRAQ data is available in the online Supplemental Table 2. Ratios are expressed as heart/liver.
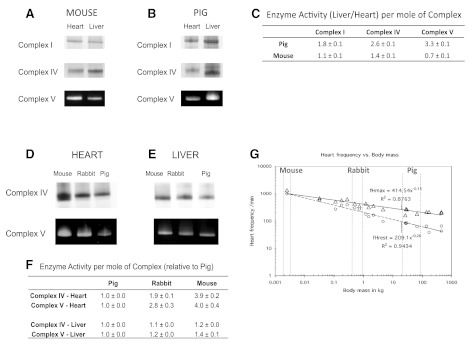
We next used optical spectroscopy to determine the absolute concentration of cyto a and estimate the metabolic stress in heart and liver tissue homogenates from the pig, rabbit, and mouse (Table 2). Similar to isolated mitochondria, the cyto a content in total tissue homogenates was significantly higher in the heart than liver for all species. Notably, while the cyto a content from the heart was nearly constant across species, the liver cyto a content decreased with increasing animal size. To calculate the metabolic stress across these tissues and species, the cyto a measurements were combined with resting respiratory rates obtained from the literature. For pig and rabbit tissues, the resting respiratory rates were obtained from in vivo data, whereas mouse data were extrapolated from in vitro preparations (specific references are provided in Table 2). Even though the cyto a content in the heart is similar between the mouse and pig, the mouse heart has an approximately sevenfold higher resting respiratory rate (69, 83), which translates to a higher cyto a catalytic rate and thus a higher resting metabolic stress. In contrast, the cyto a content in liver increases proportionally with the resting metabolic rate, which results in only a modest increase in catalytic rate and resting metabolic stress in liver across species. Collectively, these data show that the resting metabolic stress of the heart increases with decreasing body size, whereas the liver resting metabolic stress is generally higher and constant as a function of body size.
Table 2.
Cyto a contents and estimated cytochrome rates at rest for liver and heart in allometric series
| Animal | Wt, kg | Resting Heart MV̇O2, μmol O2•min−1•g wet wt−1 | Resting Liver MV̇O2, μmol O2•min−1•g wet wt−1 | Heart Cyto a content (n), nmol/g wet wt | Liver Cyto a Content (n), nmol/g wet wt | Heart Cyto a Turnover, μmol O2•nmol cyto a−1•min−1 | Liver Cyto a Turnover, μmol O2•nmol cyto a−1•min−1 | Heart Relative Metabolic Stress | Liver Relative Metabolic Stress |
|---|---|---|---|---|---|---|---|---|---|
| Mouse | 0.02 | 14a | 5.6b | 35.2 ± 1.0 (3) | 24.7 ± 3.0 (3) | 0.4 | 0.22 | 1.2 | 0.9 |
| Rabbit | 2.5 | 4.5c | 2.0d | 33.2 ± 0.6 (4) | 12.4 ± 1.6 (4) | 0.14 | 0.15 | 0.4 | 1.2 |
| Pig | 50 | 2e | 1.2b | 31 ± 0.5 (5) | 9.8 ± 0.7 (5) | 0.07 | 0.12 | 0.2 | 1.2 |
Persistent regulation of the resting activities of Complexes I, IV, and V: higher in the liver than the heart and scale inversely with animal size.
To test the hypothesis that MOPCs activity is regulated to match the energy metabolic stress, the molar activity of Complexes I, IV, and V were determined using native gel assays (74). Since the heart and liver have different amounts of MOPC per milligram of mitochondria, MOPC content was normalized to cyto a content. Identical complex loading was confirmed using the Coomassie blue-stained MOPCs band intensities.
Consistent with the metabolic stress data presented in Table 2, the activities of mouse heart and liver Complexes I, IV, and V are similar but severalfold higher in the pig liver compared with the pig heart (Fig. 4, A–C). Further evidence for the notion that MOPCs activities parallel metabolic stress is provided by the allometric series results. Specifically, the activities of Complexes IV and V in the heart increase with decreasing animal size, which correlates with increased metabolic stress (Fig. 4D). In contrast, the activities of Complexes IV and V in the liver are similar across species (Fig. 4E), reflecting the relatively constant metabolic stress of liver. Based on the partial enzymatic reactions examined using these native gel assays, these data indicate that Complex V from the resting pig heart has the largest inhibited activity (>4-fold) compared with the other MOPCs assayed. In other words, Complex V has the largest apparent reserve capacity for generating ATP during increased workloads.
Fig. 4.
Mitochondrial oxidative phophorylation complex (MOPC) activity in the heart and liver and as an allometric series. BN-PAGE activities of Complexes I, IV, and V in the heart and liver in mouse (A) and pig (B) are shown, where the signal intensity is proportional to Complex activity. C: quantitative liver/heart enzyme activity per mole of Complex (n = 6). Complex IV and V activity as a function of allomtery are shown in the heart (D) and liver (E), with normalized activities referenced to pig (n = 4) (F). G: plot adapted from Hoppeler et al. (36) where the resting (circles) and maximum (triangles) cardiac heart rates are shown as a function of animal size.
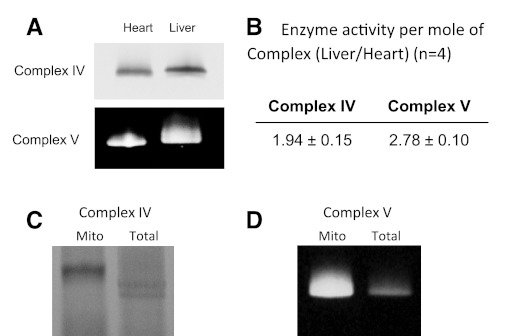
Heart and liver mitochondria reside in different cellular environments, which could alter MOPCs PTMs artificially during the isolation process. To minimize this effect, we obtained in vivo biopsy samples and analyzed them via BN-PAGE with minimal processing. Consistent with the isolated mitochondrial data presented in Fig. 2, the activity of Complexes IV and V in resting pig liver biopsies was increased by 1.9 ± 0.2-fold and 2.8 ± 0.1 (n = 4)-fold, respectively, relative to the heart (Fig. 5). Notably, the absolute reaction rates of Complexes IV and V were two to four times higher in isolated mitochondria than biopsy samples from the same animal (Fig. 5, C and D). This result suggests that the putative inhibitory PTMs associated with these MOPCs are labile, similar to what has previously been shown for PDH phosphorylation (71) and other protein phosphorylation sites (3).
Fig. 5.
Complex IV and Complex V activity from the pig heart and liver. Total tissue biopsies, 3 from each tissue, were taken from anesthesized pigs, processed, and analyzed by CN-PAGE. A: representative CN-PAGE images from this series, with the quantitative data presented in B. A comparison of Complex IV (C) and Complex V (D) activity from isolated mitochondria pig heart mitochondria and total tissue biopsies is also shown.
Effect of acute changes in metabolic stress on Complex V activity in the pig heart in vivo and the perfused rabbit heart.
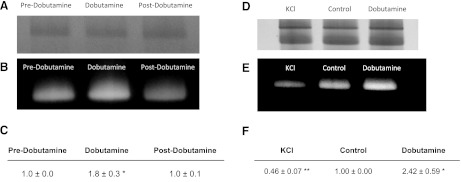
Our hypothesis predicts that the activities of MOPCs are dependent on metabolic stress and therefore acutely track metabolic stress changes to maintain the metabolic homeostasis. To test this notion, we evaluated the impact of acute changes in workload on cardiac Complex V activity in biopsy samples. Because of material limitations, we opted to study Complex V activity since the above studies showed that it had the largest reserve capacity for ATP production (Fig. 4). To assess the acute changes to Complex V activity with workload, in vivo biopsies from the pig heart were collected before, during, and after dobutamine-stimulation, and Complex V activity was determined by BN-PAGE. The dobutamine-induced increase in cardiac workload resulted in a proportional and reversible increase in Complex V activity (Fig. 6). On average, dobutamine increased the rate-pressure product by a ratio of 2.4 ± 0.6 compared with control, with a corresponding increase in Complex V activity of 1.8 ± 0.3.
Fig. 6.
Complex V activity from hearts at different metabolic stresses. First column of panels is from the pig heart, in vivo. A: Coomassie-stained bands from Complex V. B: Complex V activity in control (predobutamine), dobutamine, and postdobutamine. C: quantitative summary of data. Second column is from the perfused rabbit heart. D: Coomassie-stained bands from Complex V. E: Complex V activity after KCl infusion, Control, and dobutamine infusion. F: quantitative summary.
To generate a larger range of metabolic stress, we measured Complex V activity in the perfused rabbit heart during KCl arrest (minimal work), control, and dobutamine-stimulation conditions. Complex V increased proportionally with increasing metabolic stress in these hearts (Fig. 6, A–C). As observed for the in vivo pig heart studies detailed above, Complex V activity in the perfused rabbit heart increased by 2.4 ± 0.6-fold with dobutamine stimulation, with an increase in heart rate of 3.4 ± 0.5-fold. KCl arrest decreased Complex V activity to 0.5 ± 0.1-fold of the control rate (Fig. 6, D–F). Thus these studies attained a dynamic range of in vitro Complex V activity (from KCl arrest to dobutamine stimulation) that exceed fivefold. It should be noted that the peak metabolic activity in saline-perfused hearts is likely restricted by oxygen delivery and limited afterload, which likely underestimates the peak cardiac workload. Nonetheless, these studies demonstrate that Complex V activity is acutely modulated by acute changes to cardiac metabolic stress and that these activity changes persist through BN-PAGE processing.
A role for posttranslational modifications in modulating Complex V activity.
The current study revealed that per mole the baseline Complex V activity is approximately fourfold higher in the pig liver than the heart. This activity difference persisted throughout tissue processing and isolation of Complex V. The protein sequences for Complex V from the heart and liver vary only by an aspartate residue on the COOH-terminus of the γ-subunit (56). Thus we reasoned that the source of the Complex V activity difference between tissues resulted from 1) different stoichiometry for the subunits of Complex V, 2) tissue-specific protein/metabolite interactions, or 3) covalent posttranslational modifications, including phosphorylation, oxidation, ADP-ribosylation, etc.
The subunit composition of MOPCs between the heart and liver was evaluated using iTRAQ and SDS-electrophoresis of Complexes I, IV, and V following extraction from BN-PAGE gels (an example for Complex V is shown in Fig. 7a). No significant differences in the subunit compositions were identified for any MOPC in the pig or mouse. iTRAQ was also used to screen for tissue-specific differences in small regulatory proteins across the MOPCs; no variations were identified. Further attention was paid to Complex V and its inhibitory protein IF1. Western blotting was used to assess the content of IF1, which was found to be slightly higher in the liver than heart (Fig. 7b), negating its role in augmenting Complex V activity in the liver. Importantly, for the interaction of a small regulatory protein to increase Complex V activity by approximately fourfold, it would have to approach a stoichiometric level with the Complex (68); such an abundant protein would be easily detectable with mass spectrometry or Western blotting techniques. From these analyses we do not believe that small protein interactions or differences in subunit stoichiometry can explain the increase in Complex V activity between the pig liver and heart.
Fig. 7.
Complex V subunits and in situ 32P labeling. A: one-dimensional gel electrophoresis of isolated Complex V. B: Western blot for IF1 protein. C: 2-dimensional gel electrophoresis of isolated Complex V stained with Coomassie blue. Zoomed in autoradiograms of 32P-labeled heart and liver Complex V subunits resolved by 2D gels are the following: β (D) α (E), d-chain (F), and γ (G).
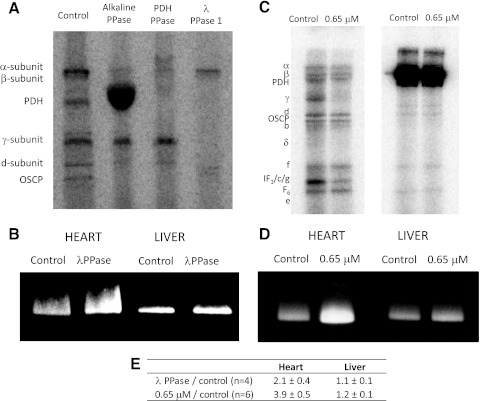
We next focused on the role of covalent posttranslational modifications in regulating MOPC activity between tissues. Since previous studies have shown distinct 32P-labeling patterns for heart and liver mitochondria (4) and the current study revealed the largest activity difference for Complex V, we evaluated 32P-labeling in Complex V isolated from pig heart and liver mitochondria. As shown in Fig. 7C, most Complex V subunits were resolved by 2D SDS gel electrophoresis. In heart mitochondria, 32P-labeling was observed for the α-, β- γ-, and d-chain subunits of Complex V (Fig. 7, D–G). By comparison, in the liver the γ-subunit was virtually dephosphorylated, whereas the d-chain was weakly phosphorylated. Most studies on the functional role of protein phosphorylation have found this modification to be inhibitory. Consistent with this notion, our findings suggest that the phosphorylation of several Complex V subunits in the heart may be responsible for its decreased activity relative to the liver. To directly correlate decreased activity in cardiac Complex V with phosphorylation status, we conducted a phosphatase screen. λ-Phosphatase was found to dephosphorylate the γ-subunit of Complex V in hearts, which resulted in more than a twofold increase in Complex V activity (Fig. 8, A and B). To ensure that the λ-phosphatase was not altering cardiac Complex V activity indirectly, we also compared the effect of λ-phosphatase on liver Complex V. Since the γ-subunit is inherently dephosphorylated in the liver, no increase in its Complex V activity was expected; this finding was confirmed (Fig. 8B). Importantly, these phosphatase studies also show that the difference in Complex V activity between the heart and liver are due to reversible covalent modifications and not merely irreversible damage that may have occurred during tissue processing.
Fig. 8.
Effect of phosphatases and Ca2+ on the 32P-labeling and activity of Complex V. A: 32P-labeled Complex V incubated with difference phosphatases. B: effect of λ-phosphatase on pig heart and liver Complex V activity. Effect of 650 nM free Ca2+ on the 32P-labeling (C) and activity of Complex V (D) in heart and liver mitochondria (Ca2+ incubation done in intact mitochondria, before Complex V isolation). E: quantitative summary.
Previous studies have also shown that heart mitochondria are dependent on Ca2+ for maximum activation (31, 61, 84). Utilizing this physiological condition, we correlated the 32P-labeling profiles of Complex V under control (0 μM free Ca2+) and activating Ca2+ levels (0.65 μM free Ca2+) with activity in the heart and liver. When intact heart mitochondria were incubated with Ca2+, the γ-subunit and the band containing the IF1, c, and g-subunits were dephosphorylated, which correlated with a 3.9 ± 0.5-fold increase in Complex V activity (Fig. 8, C and D). Similar to the phosphatase studies, Ca2+ incubation did not alter the phosphorylation status or activity of Complex V in the liver. It is important to point out that these Ca2+ effects were only observed when the Ca2+ incubation was performed in intact heart mitochondria (i.e., no changes in 32P-labeling or activity were shown when isolated Complex V was incubated with Ca2+). This finding suggests that Ca2+ is regulating processes, perhaps kinases and phosphatases, which reside in and require an intact mitochondrion.
DISCUSSION
In this study, proteomics, biochemical, and physiological approaches were combined to demonstrate that the molar activities of MOPCs scale with metabolic stress in the liver and heart for a given animal or across an allometric series. In addition, we demonstrate that the activity of Complex V is acutely modulated by cardiac workload and associated changes in metabolic stress. These data are consistent with the regulation of net MOPCs flux capacity by modulating protein content relative to the maximum energy conversion requirements of tissues (87). In tissues with low metabolic dynamic ranges such as the liver, MOPCs are operating at a relatively constant high metabolic stress and the changes in resting metabolic rate with body size are matched with mitochondrial mass consistent with previous studies (48, 69). In tissue with large dynamic ranges in energy conversion, such as the heart, the mitochondria content is held relatively constant while the activity of the MOPCs is acutely modulated in proportion to the metabolic stress. In these dynamic tissues, we propose that PTMs provide a mechanism for reserving a significant fraction of MOPCs activity while the tissue is “rest.” During increases in energy conversion, alterations in PTM switch on the MOPCs to a more active form. This model of modulating MOPCs activity with work requirements parallels the previously demonstrated modulation of PDH activity delivering reducing equivalents to the cytochrome chain with work. The modulation of both the delivery of reducing equivalents and the MOPCs converting this energy into ATP provides a provides a theoretically balanced activation of energy conversion during work transitions.
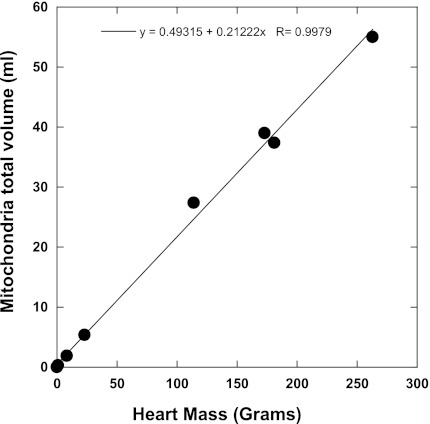
Despite the large difference in resting metabolic rates between the pig and mouse heart, the current study found that the mitochondrial content is fixed at ∼32 nmol cyto a/g wet wt across species. This translates to mitochondria composing ∼21% of total tissue protein of the heart cell [assuming 140 mg of cellular protein/g wet wt (13) and ∼1 nmol cyto a/mg mito protein; see results]. These data appear to conflict with an earlier study by Hoppeler et al. (36), which concluded cardiac mitochondrial volume scaled with resting metabolic rate. However, replotting mitochondrial volumes by Hoppeler et al. as a function of heart mass (Fig. 9) results in a linear plot with a slope of 0.21 (0.22 if horse and cattle are included) consistent with our results. A similar analysis of Hoppeler's data was made by Dobson et al. (25). These data demonstrate that the maximum aerobic capacity of the heart is nearly identical across species, which suggests that there is an optimal balance of mitochondrial and contractile elements in mammalian heart cells. A similar conclusion was recently reached in proteomic studies on the right and left ventricles, which showed that despite large differences in workload, the protein composition per myocyte was nearly identical between ventricles (66, 75).
Fig. 9.
Volume of mitochondria per heart muscle mass across an allometric series. These data were adapted from Hoppeler et al. (36) and reveals that mitochondrial volume is fixed at ∼21% in cardiac cells across species. Benson (13) found that 14% of the wet weight of the heart is noncollagen protein. This corresponds to 140 mg of cellular protein per gram of heart wet weight. With the use of the volume fraction of 21% and assuming a similar density of protein across different cell compartments, a value of ∼30 mg of mitochondria protein per gram of heart is calculated. Since there is ∼1 nmole cyto a/mg mitochondria protein (see results), the data from Hoppeler et al. (36) predicts a cyto a content of ∼30 nmole cyto a/gram of heart, which is in good agreement with the values presented in Table 2.
In contrast to the fixed mitochondrial content of the heart, the mitochondrial content of the liver scales inversely with animal size. This finding agrees with the previous studies on the liver (48, 69). Since liver lacks a dynamic range for ATP production, its resting state does not require a large energetic reserve capacity or the need for a large dynamic regulation of MOPCs activity. Instead, these data imply that MOPCs and mitochondria content are elevated in the small animal liver to meet the increased energy conversion at a relatively fixed metabolic stress. We suggest that liver is able to increase its mitochondrial content since it does not have the spatial constraints of the cardiac contractile requirements.
The current study suggests that the maximum activity of Complex V is modulated directly by, or in concert with, the metabolic stress. We show that the total activity of Complex V matches the resting metabolic stress of the heart and liver across an allometric series as well as during acute changes in metabolic stress in the dynamic heart. Modulating Complex V maximum activity, either through protein content or PTM effects, allows the rate of ATP generation to be matched with ATP consumption in the metabolic homeostasis with essentially constant ADP and Pi or ΔGATP. This is done by essentially modulating the effective maximum velocity of the reaction permitting an increase in flux with a constant substrate or thermodynamic driving force. Again, the maximum activity of Complex V can be modulated via protein content or the activity per mole. The content of Complex V must match the maximum energy conversion requirements to support this activity. In tissues with large energy conversion dynamic ranges, the molar activity of the Complex V seems to be additionally scaled to match the changing metabolic stress, presumably becoming fully active at the maximum energy conversion rates. Consistent with this later notion, we demonstrated that extracted Complex V activity was persistently inhibited in the resting heart relative to the liver but was able to be acutely activated in response to a dobutamine-induced increase in energy conversion requirements associated with a stimulation of inotrophy and heart rate. Previous studies have demonstrated that this type of stimulation does not alter the ADP, Pi, or ΔGATP of the heart in vivo (42), consistent with the maintenance of the metabolic homeostasis. Based on these results, we speculate that kinetic regulation of Complex V activity is fundamental to the maintenance of the energetic homeostasis both chronically and acutely. Specifically, we propose that PTMs provide a mechanism for altering ATP production at the level of the oxidative phosphorylation complexes of oxidative phosphorylation to match metabolic stress, without changing the conventional driving forces (i.e., ΔΨ and ΔGATP).
The fact that the changes in MOPCs activity persisted through the native gel isolation process is the best evidence that the activity of the enzyme was modulated by covalent PTMs or other strong associations. Persistence through isolation was also one of the earliest observations in the evaluation of PDH activity modulation by insulin action (22). While protein/metabolite associations or tissue-specific MOPCs subunit differences could also explain the persistence of MOPC activity differences, no such findings were identified in this study. Several PTMs have been shown to occur in matrix proteins, including the identification of phosphorylation sites for Complex IV (1, 26, 51, 81) and Complex V (3, 4, 41). Our laboratory recently showed that the 32P-labeling patterns in pig heart and liver mitochondria are very different, independent of the protein concentration (4). Herein we focus on the differential phosphorylation of Complex V, which is persistent and reversible and therefore consistent with the criteria for a regulatory PTMs. Comparing tissues, 32P-labeling in the γ- and d-chain subunits is lower in the liver compared with the heart. Consistent with these events being inhibitory in the heart, we show that λ-phosphatase and Ca2+ treatments dephosphorylate the γ-subunit and activate Complex V. While the phosphorylation sites in MOPCs may be similar to bacterial autophosphorylation process, our previous work on this topic did not reveal autophosphorylation for the γ-subunit (67), suggesting that it is regulated by a matrix kinase or phosphatase system. While further work is required to determine the actual phosphorylation site(s) for these Complex V subunits, their direct impact on activity, and the associated matrix kinases/phosphatase, the large activity differences revealed in this study provides a unique opportunity to differentially identify PTMs that dynamically influence MOPCs function.
Though skeletal muscle has an even larger metabolic dynamic range than the heart approaching 100-fold (34), we opted not to include this tissue in this study. We have found that the heterogeneity in the fiber type composition (16), remodeling by chronic exercise (35), and reliance on anaerobic ATP production in fast twitch fibers complicates the interpretation of biopsy data. In contrast, heart and liver are relatively homogenous in their composition even across species and highly dependent on mitochondrial oxidative phosphorylation. In addition, skeletal muscle exists as a continuum of different fiber types with different metabolic and functional capacities across species; thus, normalization across an allometric series would be difficult.
Using native gel electrophoresis, derived mitochondria complex activities is problematical in extrapolating to tissue mitochondrial function and discussion of this study limitation is warranted. Although most of the proteins believed to be associated with a given complex persist through the isolation process, the complex finds itself in a remarkably nonphysiological condition: the complex is outside of the membrane with variable amounts of associated lipid and without a ∼180 mV ΔΨ, which likely plays a major role in controlling fluxes through Complexes I, III, IV, and V. For example, isolated Complex I directly interacts with oxygen, which reacts with NADH to produce large amounts of ROS under these native gel conditions (15). Geometric relationships within the cristae and between complexes (see Zick, 2009 ZICK2009/id and Lenaz, 2007 LENAZ2007/id) that could dynamically modulate complex activity is eliminated and not evaluated by this approach. The substrate and metabolite milieu is also altered with some metabolites having very tight associations such as Mg-ADP in Complex V that has inhibitory effects (62). In addition, these in gel assays only sample partial reactions (Complexes I) or the reverse reaction (Complex V) in the absence of a ΔΨ. Thus these activities, which are extensively reported in the literature, really represent rough approximations of the in vivo enzyme activities. Another concern is that these assays are simply monitoring differential damage of the complexes during the isolation procedures. This is likely happening to some extent; however, we found that that MOPCs catalytic rate increased with processing of the samples, not decreased (see Fig. 5). Thus, if anything, the isolation is not damaging the complexes but removing inhibitory factors. Despite these drawbacks, reversible changes in enzyme activity, for example, in the pig heart in vivo (see Fig. 6), that persist throughout the BN- or CN-PAGE process imply that the complex has been rather significantly modified and likely reflects alteration to the enzyme activity under normal physiological conditions. This is particularly true for Complex V where the rotation of the γ-subunit is required for both synthetic and hydrolytic activity.
Another approach would be to isolate intact mitochondria from the different tissues and “probe” the different complex activities using different substrates and inhibitors (for example see Ref. 11). This is a common approach used by many in the field, including this laboratory. However, we have found that the mitochondrial isolation process (i.e., in situ perfused, ionic composition of initial isolation solutions, etc.) and the conditions selected for performing the experiments (i.e., carbon substrates and ion concentrations, including calcium, magnesium, sodium, etc.) all change the relative metabolic poise of this isolated organelle, making almost any result possible. This is particularly problematic for the carbon substrates used since each tissue is programmed differently for using different substrates (38). For example, Randle's laboratory (43) first showed that the activity of PDH in mitochondria is much different immediately after isolation than after several minutes of rewarming in a “physiological” medium. We confirmed this phenomenon for many phosphorylation sites in the mitochondrial matrix by direct 32P-labeling; the largest fraction of 32P incorporation, including PDH, was found to occur during the rewarming period, which established a new, nonexchanging, steady state (4). These data imply that the phenomenon described by Randle et al. (71) for PDH is potentially occurring in many other PTM proteins. Indeed, we find a striking activation of complex activity in fully isolated mitochondria compared with rapid tissue biopsy samples (Fig. 5) or gross tissue homogenates. Additionally, we also have shown that Complex V activity in isolated mitochondria is sensitive to calcium. To observe a calcium activation for Complex V, calcium chelators (i.e., EGTA) are required in the initial tissue perfusion, and a calcium depletion step must be performed in the isolated mitochondria (84). The best way to maintain the matrix calcium at physiological levels in isolated mitochondria preparations has been a long-standing controversy, with no gold standard in vivo to standardize to. It should also be noted that even an RCR of 10 is still much lower than the change in respiratory rate from an arrested heart to the maximum rate of respiration, which likely approaches 100. Thus the mitochondria are not as coupled in vitro as in the intact tissue. Taking these limitations into account, we decided that the direct isolation of MOPCs from tissues using BN-PAGE or CN-PAGE, which keeps the complexes intact and minimizes in vitro incubation conditions, was the best available methodology to evaluate our hypothesis.
Perspectives and Significance
In this study proteomics, biochemical, and physiological strategies were combined to evaluate the relationship between MOPCs content and activity in the liver and heart of an allometric series of animals and during work transitions in the heart. These studies reveal two different organ strategies in adapting to higher resting energy conversion rate with decreasing body weight. In the liver, with a very low energy metabolism dynamic range and associated high net metabolic stress, the MOPCs content scaled with the resting metabolic rate and maximum energy conversion rate. In the heart, the MOPCs content and apparent maximum energy conversion rate is relatively constant as a function of body size while the dynamic range of energy conversion decreases dramatically with decreasing body size. This later result suggests that the ratio of MOPC and contractile elements in the heart is nearly constant as a function of body size. This implies that this ratio is optimized with regard to the use of cellular volume to perform work and mitochondrial energy conversion rate in the mammalian heart. Though the heart MOPCs content was constant as a function of body weight, we found that MOPCs activity was proportional to the resting metabolic stress, across the allometric series, as well as during step increases in work in the porcine and rabbit heart. The persistent nature of MOPCs activity changes in the isolated complexes is consistent with PTMs reversibly modulating MOPCs activity. We demonstrate that protein phosphorylation in Complex V may contribute to the acute regulation of this complex; however, the specific phosphorylation sites and kinase-phosphatase system is unknown. We speculate that this alteration in maximum velocity of MOPCs activity is important in maintaining the metabolic homeostasis of the heart, both as a function of body size as well as during the work transitions in larger animals. These studies suggest that the modulation of MOPCs content and activity is critical for mammalian tissues to maintain their metabolic homeostasis as a function of body size as well as during dynamic changes in metabolic activity. The direct modulation of MOPCs activity by PTMs in response changes in metabolic stress is a novel hypothesis and may be extended to other tissues with large dynamic ranges in workload, including skeletal muscle, brain, and kidney. Whether compromises of the metabolic regulation of MOPCs activity with metabolic stress are important in different disease states of man is also unknown.
GRANTS
This work was supported by the National Institutes of Health Division of Intramural Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.P. and R.S.B. conception and design of research; D.P., R.C., A.M.A., B.G., J.t., D.J.C., and R.S.B. performed experiments; D.P., R.C., A.M.A., B.G., J.t., D.J.C., and R.S.B. analyzed data; D.P., R.C., A.M.A., B.G., D.J.C., and R.S.B. interpreted results of experiments; D.P., A.M.A., B.G., J.t., and R.S.B. prepared figures; D.P., D.J.C., and R.S.B. drafted manuscript; D.P., R.C., A.M.A., B.G., D.J.C., and R.S.B. edited and revised manuscript; D.P., R.C., A.M.A., B.G., J.t., D.J.C., and R.S.B. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the early observations of Complex V phosphorylation differences in liver and heart by Dr. Rachel Hopper in our laboratory using protein phosphorylation sensitive dyes and subsequent discussions on the differences in this protein in these two tissues.
Present address of D. Phillips: Harvard Medical School, 260 Longwood Ave., Room 233, Boston, MA 02115.
REFERENCES
- 1. Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab 13: 712–719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105: 14447–14452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aponte AM, Phillips D, Harris RA, Blinova K, French S, Johnson DT, Balaban RS. 32P labeling of protein phosphorylation and metabolite association in the mitochondria matrix. Methods Enzymol 457: 63–80, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aponte AM, Phillips D, Hopper RK, Johnson DT, Harris RA, Blinova K, Boja ES, French S, Balaban RS. Use of (32)P to study dynamics of the mitochondrial phosphoproteome. J Proteome Res 8: 2679–2695, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol 34: 1259–1271, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Balaban RS. Maintenance of the metabolic homeostasis of the heart: developing a systems analysis approach. Ann NY Acad Sci 1080: 140–153, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Balaban RS. Domestication of the cardiac mitochondrion for energy conversion. J Mol Cell Cardiol 46: 832–841, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balaban RS. The role of Ca(2+) signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta 1787: 1334–1341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balaban RS, Mootha VK, Arai A. Spectroscopic determination of cytochrome c oxidase content in tissues containing myoglobin or hemoglobin. Anal Biochem 237: 274–278, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Bao J, Sack MN. Protein deacetylation by sirtuins: delineating a post-translational regulatory program responsive to nutrient and redox stressors. Cell Mol Life Sci 67: 3073–3087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benard G, Faustin B, Passerieux E, Galinier A, Rocher C, Bellance N, Delage JP, Casteilla L, Letellier T, Rossignol R. Physiological diversity of mitochondrial oxidative phosphorylation. Am J Physiol Cell Physiol 291: C1172–C1182, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Bender E, Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett 466: 130–134, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Benson ES. Composition and state of protein in heart muscle of normal dogs and dogs with experimental myocardial failure. Circ Res 3: 221–228, 1955 [DOI] [PubMed] [Google Scholar]
- 14. Bisetto E, Di PF, Simula MP, Mavelli I, Lippe G. Mammalian ATPsynthase monomer versus dimer profiled by blue native PAGE and activity stain. Electrophoresis 28: 3178–3185, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Blinova K, Levine RL, Boja ES, Griffiths GL, Shi ZD, Ruddy B, Balaban RS. Mitochondrial NADH fluorescence is enhanced by complex I binding. Biochemistry 47: 9636–9645, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buller AJ, Eccles JC, Eccles RM. Differentiation of fast and slow muscles in the cat hind limb. J Physiol 150: 399–416, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen R, Fearnley IM, Peak-Chew SY, Walker JE. The phosphorylation of subunits of Complex I from bovine heart mitochondria. J Biol Chem 279: 26036–26045, 2004 [DOI] [PubMed] [Google Scholar]
- 18. ConstantinTeodosiu D, Carlin J, Cederblad G, Harris R, Hultman Acetyl group accumulation and pyruvate dehydrogenase activity in human muscle during incremental exercise. Acta Physiologica Scand 143: 367–372, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Das AM, Harris DA. Reversible modulation of the mitochondrial ATP synthase with energy demand in cultured rat cardiomyocytes. FEBS Lett 256: 97–100, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787: 1309–1316, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Denton RM, McCormack JG. The calcium sensitive dehydrogenases of vertebrate mitochondria. Cell Calcium 7: 377–386, 1986 [DOI] [PubMed] [Google Scholar]
- 22. Denton RM, McCormack JG, Marshall SE. Persistence of the effect of insulin on pyruvate dehydrogenase activity in rat white and brown adipose tissue during the preparation and subsequent incubation of mitochondria. Biochem J 217: 441–452, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Denton RM, Randle PJ, Bridges BJ, Cooper RH, Kerbey AL, Pask HT, Severson DL, Stansbie D, Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem 9: 27–53, 1975 [DOI] [PubMed] [Google Scholar]
- 24. Denton RM, Randle PJ, Martin BR. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J 128: 161–163, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dobson GP, Himmelreich U. Heart design: free ADP scales with absolute mitochondrial and myofibrillar volumes from mouse to human. Biochim Biophys Acta 1553: 261–267, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Fang JK, Prabu SK, Sepuri NB, Raza H, Anandatheerthavarada HK, Galati D, Spear J, Avadhani NG. Site specific phosphorylation of cytochrome c oxidase subunits I, IVi1 and Vb in rabbit hearts subjected to ischemia/reperfusion. FEBS Lett 581: 1302–1310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forner F, Foster LJ, Campanaro S, Valle G, Mann M. Quantitative proteomic comparison of rat mitochondria from muscle, heart, and liver. Mol Cell Proteomics 5: 608–619, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab 10: 324–335, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Fowler MD, Ryschon TW, Wysong RE, Combs CA, Balaban RS. Normalized metabolic stress for 31P-MR spectroscopy studies of human skeletal muscle: MVC vs. muscle volume. J Appl Physiol 83: 875–883, 1997 [DOI] [PubMed] [Google Scholar]
- 30. French SA, Territo PR, Balaban RS. Correction for inner filter effects in turbid samples: fluorescence assays of mitochondrial NADH. Am J Physiol Cell Physiol 275: C900–C909, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Harris DA, Das AM. Control of mitochondrial ATP synthesis in the heart. Biochem J 280: 561–573, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heineman FW, Balaban RS. Effects of afterload and heart rate on NAD(P)H redox state in the isolated rabbit heart. Am J Physiol Heart Circ Physiol 264: H433–H440, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Hochachka PW, McClelland GB. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol 200: 381–386, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Hochachka PW, McClelland GB. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol 200: 381–386, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967 [PubMed] [Google Scholar]
- 36. Hoppeler H, Lindstedt SL, Claassen H, Taylor CR, Mathieu O, Weibel ER. Scaling mitochondrial volume in heart to body mass. Respir Physiol 55: 131–137, 1984 [DOI] [PubMed] [Google Scholar]
- 37. Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry 45: 2524–2536, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson DT, Harris RA, Blair PV, Balaban RS. Functional consequences of mitochondrial proteome heterogeneity. Am J Physiol Cell Physiol 292: C698–C707, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Johnson DT, Harris RA, French S, Aponte A, Balaban RS. Proteomic changes associated with diabetes in the BB-DP rat. Am J Physiol Endocrinol Metab 296: E422–E432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson DT, Harris RA, French S, Blair PV, You J, Bemis KG, Wang M, Balaban RS. Tissue heterogeneity of the mammalian mitochondrial proteome. Am J Physiol Cell Physiol 292: C689–C697, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Kane LA, Youngman MJ, Jensen RE, Van Eyk JE. Phosphorylation of the F(1)F(o) ATP synthase beta subunit: functional and structural consequences assessed in a model system. Circ Res 106: 504–513, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katz LA, Swain JA, Portman MA, Balaban RS. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol Heart Circ Physiol 256: H265–H274, 1989 [DOI] [PubMed] [Google Scholar]
- 43. Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J 154: 327–348, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khvorostov I, Zhang J, Teitell M. Probing for mitochondrial complex activity in human embryonic stem cells. J Vis Exp 17: 724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kimmig R, Mauch TJ, Kerzl W, Schwabe U, Scholz R. Actions of glucagon on flux rates in perfused rat liver. 1 Kinetics of the inhibitory effect on glycolysis and the stimulatory effect on glycogenolysis. Eur J Biochem 136: 609–616, 1983 [DOI] [PubMed] [Google Scholar]
- 46. Kobayashi K, Neely JR. Mechanism of pyruvate dehydrogenase activation by increased cardiac work. J Mol Cell Cardiol 15: 369–382, 1983 [DOI] [PubMed] [Google Scholar]
- 47. Kojic Z, Scepanovic L, Popovic N. Myocardial oxygen consumption regulation in isolated mouse heart: assessment by intracoronary administration of exogenous nitric oxide. Acta Physiol Hung 93: 263–270, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Krebs HA. Body size and tissue respiration. Biochim Biophys Acta 4: 249–269, 1950 [DOI] [PubMed] [Google Scholar]
- 49. Krieg RC, Knuechel R, Schiffmann E, Liotta LA, Petricoin EF, Herrmann PC. Mitochondrial proteome: Cancer-altered metabolism associated with cytochrome c oxidase subunit level variation. Proteomics 4: 2789–2795, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Lai Y, Chen Y, Watkins SC, Nathaniel PD, Guo F, Kochanek PM, Jenkins LW, Szabo C, Clark RSB. Identification of poly-ADP-ribosylated mitochondrial proteins after traumatic brain injury. J Neurochem 104: 1700–1711, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Lee I, Bender E, Kadenbach B. Control of mitochondrial membrane potential and ROS formation by reversible phosphorylation of cytochrome c oxidase. Mol Cell Biochem 234–235: 63–70, 2002 [PubMed] [Google Scholar]
- 52. Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Huttemann M. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem 280: 6094–6100, 2005 [DOI] [PubMed] [Google Scholar]
- 52a. Lenaz G, ML Genova. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: random collisions vs. solid state electron channeling. Am J Physiol Cell Physiol 292: C1221–C1239, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Lin TK, Hughes G, Muratovska A, Blaikie FH, Brookes PS, rley-Usmar V, Smith RA, Murphy MP. Specific modification of mitochondrial protein thiols in response to oxidative stress: a proteomics approach. J Biol Chem 277: 17048–17056, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Lindberg B, Darle N. The effect of glucagon and blood transfusion on hepatic circulation and oxygen consumption in hemorrhagic shock. J Surg Res 23: 257–263, 1977 [DOI] [PubMed] [Google Scholar]
- 55. Martini WZ, Stanley WC, Huang H, Rosiers CD, Hoppel CL, Brunengraber H. Quantitative assessment of anaplerosis from propionate in pig heart in vivo. Am J Physiol Endocrinol Metab 284: E351–E356, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Matsuda C, Endo H, Ohta S, Kagawa Y. Gene structure of human mitochondrial ATP synthase gamma-subunit. Tissue specificity produced by alternative RNA splicing. J Biol Chem 268: 24950–24958, 1993 [PubMed] [Google Scholar]
- 57. McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391–425, 1990 [DOI] [PubMed] [Google Scholar]
- 58. Montagnani M, Aldini R, Roda A, Caruso ML, Gioacchini AM, Lenzi PL, Roda E. Species differences in hepatic bile acid uptake: comparative evaluation of taurocholate and tauroursodeoxycholate extraction in rat and rabbit. Comp Biochem Physiol A Physiol 113: 157–164, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Mootha VK, Arai AE, Balaban RS. Maximum oxidative phosphorylation capacity of the mammalian heart. Am J Physiol Heart Circ Physiol 272: H769–H775, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115: 629–640, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Moreno-Sanchez R. Regulation of oxidative phosphorylation in mitochondria by external free Ca2+ concentrations. J Biol Chem 260: 4028–4034, 1985 [PubMed] [Google Scholar]
- 62. Murataliev MB. Adenine nucleotide binding at a noncatalytic site of mitochondrial F1-ATPase accelerates a Mg(2+)- and ADP-dependent inactivation during ATP hydrolysis. Biochemistry 31: 12885–12892, 1992 [DOI] [PubMed] [Google Scholar]
- 63. Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134: 112–123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pasdois P, Deveaud C, Voisin P, Bouchaud V, Rigoulet M, Beauvoit B. Contribution of the phosphorylable complex I in the growth phase-dependent respiration of C6 glioma cells in vitro. J Bioenerg Biomembr 35: 439–450, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Pettit FH, Roche TE, Reed LJ. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun 49: 563–571, 1972 [DOI] [PubMed] [Google Scholar]
- 66. Phillips D, Aponte AM, Covian R, Neufeld E, Yu ZX, Balaban RS. Homogenous protein programming in the mammalian left and right ventricle free walls. Physiol Genomics 43: 1196–1206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Phillips D, Aponte AM, Covian RG, Balaban RS. Intrinsic protein kinase activity of mitochondrial oxidative phosphorylation complexes. Biochemistry 50: 2515–2529, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Phillips D, Reilley MJ, Aponte AM, Wang G, Boja E, Gucek M, Balaban RS. Stoichiometry of STAT3 and mitochondrial proteins: Implications for the regulation of oxidative phosphorylation by protein-protein interactions. J Biol Chem 285: 23532–23536, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Porter RK, Brand MD. Cellular oxygen consumption depends on body mass. Am J Physiol Regul Integr Comp Physiol 269: R226–R228, 1995 [DOI] [PubMed] [Google Scholar]
- 70. Randle PJ. Phosphorylation-dephosphorylation cycles and the regulation of fuel selection in mammals. Curr Top Cell Regul 18: 107–129, 1981 [DOI] [PubMed] [Google Scholar]
- 71. Randle PJ, Denton RM, Pask HT, Severson DL. Calcium ions and the regulation of pyruvate dehydrogenase. Biochem Soc Symp: 75–87, 1974 [PubMed] [Google Scholar]
- 72. Reinders J, Sickmann A. Proteomics of yeast mitochondria. Methods Mol Biol 372: 543–557, 2007 [DOI] [PubMed] [Google Scholar]
- 73. Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium 26: 193–199, 1999 [DOI] [PubMed] [Google Scholar]
- 74. Schagger H, von JG. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199: 223–231, 1991 [DOI] [PubMed] [Google Scholar]
- 75. Scholten A, Mohammed S, Low TY, Zanivan S, van Veen TA, Delanghe B, Heck AJ. In-depth quantitative cardiac proteomics combining electron transfer dissociation and the metalloendopeptidase Lys-N with the SILAC mouse. Mol Cell Proteomics 10: O111.–008474., 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Scholz TD, Balaban RS. Mitochondrial F1-ATPase activity of canine myocardium: effects of hypoxia and stimulation. Am J Physiol Heart Circ Physiol 266: H2396–H2403, 1994 [DOI] [PubMed] [Google Scholar]
- 77. Schulenberg B, Goodman TN, Aggeler R, Capaldi RA, Patton WF. Characterization of dynamic and steady-state protein phosphorylation using a fluorescent phosphoprotein gel stain and mass spectrometry. Electrophoresis 25: 2526–2532, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Schwerzmann K, Hoppeler H, Kayar SR, Weibel ER. Oxidative capacity of muscle and mitochondria: Correlation of physiological, biochemical, and morphometric characteristics. Proc Natl Acad Sci USA 86: 1583–1587, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Soboll S. Regulation of energy metabolism in liver. J Bioenerg Biomembr 27: 571–582, 1995 [DOI] [PubMed] [Google Scholar]
- 81. Steenaart NA, Shore GC. Mitochondrial cytochrome c oxidase subunit IV is phosphorylated by an endogenous kinase. FEBS Lett 415: 294–298, 1997 [DOI] [PubMed] [Google Scholar]
- 82. Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res 101: 1155–1163, 2007 [DOI] [PubMed] [Google Scholar]
- 83. Taylor CR. Structural and functional limits to oxidative metabolism: insights from scaling. Annu Rev Physiol 49: 135–146, 1987 [DOI] [PubMed] [Google Scholar]
- 84. Territo PR, Mootha VK, French SA, Balaban RS. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of F0/F1ATPase. Am J Physiol Cell Physiol 278: C423–C435, 2000 [DOI] [PubMed] [Google Scholar]
- 85. Tuttle RR, Mills J. Dobutamine: development of a new catecholamine to selectively increase cardiac contractility. Circ Res 36: 185–196, 1975 [DOI] [PubMed] [Google Scholar]
- 86. von Restorff W, Holtz J, Bassenge E. Excerise induced augmentation of myocardial oxygen extraction in spite of normal dilatory capacity in dogs. Pflugers Arch 372: 181–185, 1977 [DOI] [PubMed] [Google Scholar]
- 87. Weibel ER, Taylor CR, Hoppeler H. The concept of symmorphosis: a testable hypothesis of structure- function relationship. Proc Natl Acad Sci USA 88: 10357–10361, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Weiss HR. Regional oxygen consumption and supply in the rabbit heart–effect of nitroglycerin and propranolol. J Pharmacol Exp Ther 211: 68–73, 1979 [PubMed] [Google Scholar]
- 89. Westermann B, Neupert W. “Omics” of the mitochondrion. Nat Biotechnol 21: 239–240, 2003 [DOI] [PubMed] [Google Scholar]
- 90. Wittig I, Carrozzo R, Santorelli FM, Schagger H. Functional assays in high-resolution clear native gels to quantify mitochondrial complexes in human biopsies and cell lines. Electrophoresis 28: 3811–3820, 2007 [DOI] [PubMed] [Google Scholar]
- 91. Zhao Y, Richman A, Storey C, Radford NB, Pantano P. In situ fiber-optic oxygen consumption measurements from a working mouse heart. Anal Chem 71: 3887–3893, 1999 [DOI] [PubMed] [Google Scholar]
- 92. Zick M, Rabl R, Reichert AS. Cristae formation linking ultrastructure and function of mitochondria. Biochimica et Biophysica Acta Mol Cell Res 1793: 5–19, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.