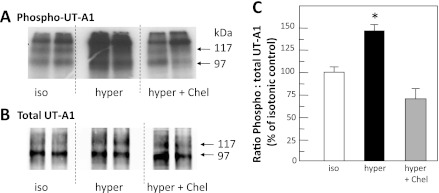
Fig. 1.
Inhibition of protein kinase C (PKC) blocks hypertonic stimulation of UT-A1 phosphorylation. Metabolically labeled (32P) rat inner medullary collecting ducts (IMCDs) were incubated with 290-mosmol/kgH2O buffer (iso), 600-mosmol/kgH2O buffer (hyper), or 600-mosmol/kgH2O buffer with 10 μM chelerythrine (hyper + Chel) and then immunoprecipitated with anti-UT-A1 and analyzed by Western blot and autoradiography. A: autoradiogram of 32P-UT-A1. B: Western blot of total UT-A1 for representative samples. Each lane provides results from the kidneys of a separate animal. Arrows indicate the 117- and 97-kDa glycoprotein forms of UT-A1. C: bar of the ratio of phosphorylated UT-A1 to total UT-A1 from all samples from 2 separate cohorts of animals, presented as means ± SE, n = 8/condition. *P < 0.05 vs. isotonic control.