Abstract
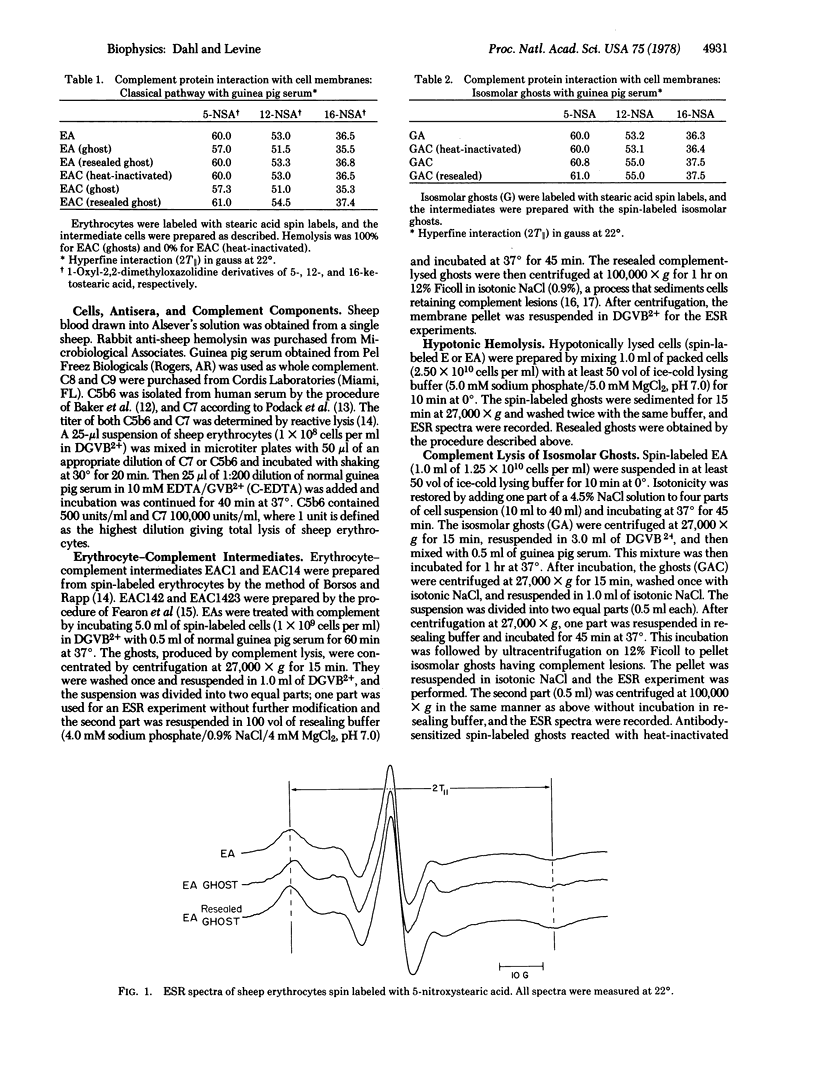
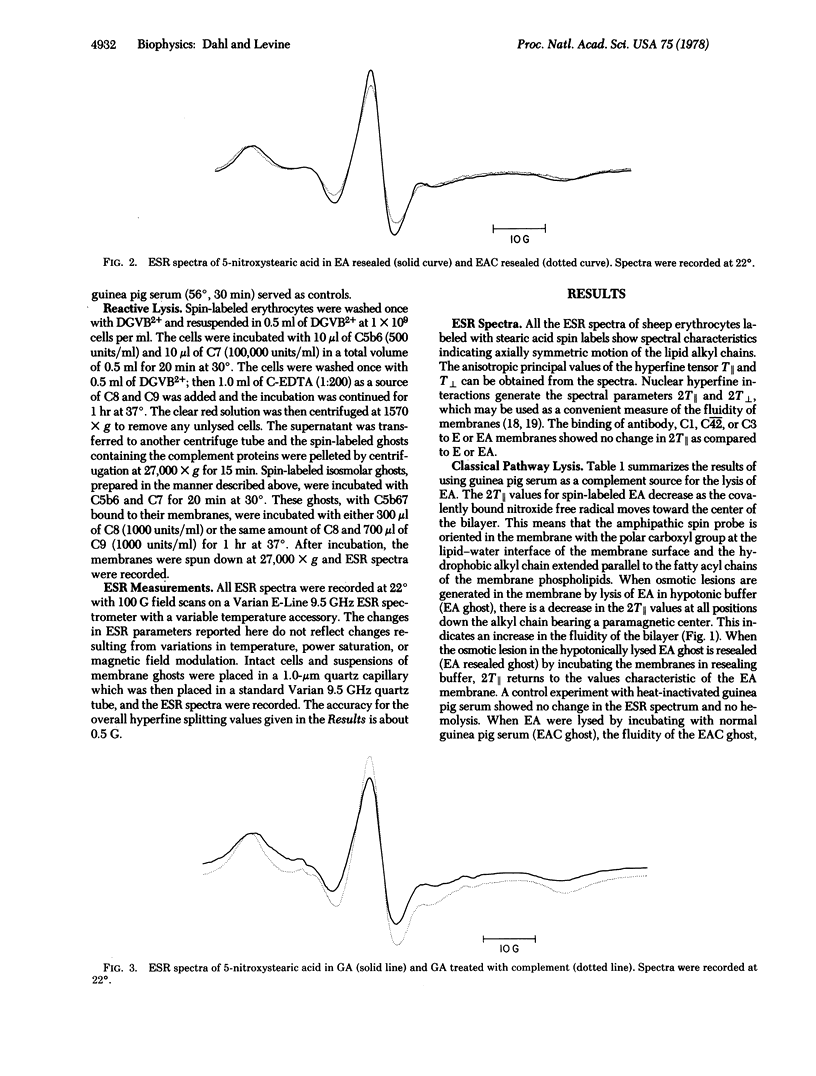
Sheep erythrocytes have been spin labeled with 5-, 12-, and 16-nitroxystearic acid in order to investigate complement-induced changes in the physical state of the lipid bilayer. Formation of osmotic lesions in the membrane causes an increase in the fluidity of the membrane which overcomes the decrease in membrane fluidity caused by the interaction of the complement proteins. A decrease in membrane fluidity is observed only when complement-lysed membranes are resealed or when complement proteins react with isosmolar ghosts that do not undergo osmotic lysis. The decrease in bulk fluidity of the membrane is first observed when C8 binds to the membranes bearing C5b67 and is enhanced upon the subsequent binding of C9. The decrease in membrane fluidity shown by the electron spin resonance spectra of spin-labeled fatty acids suggests that certain of the complement proteins penetrate the membrane and interact with hydrophobic regions of the lipid bilayer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Rubin L. G., Lint T. F., McLeod B. C., Gewurz H. Binding of the complement intermediate C56 to zymosan in acute phase human sera. Clin Exp Immunol. 1975 Apr;20(1):113–124. [PMC free article] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Immune hemolysis: a simplified method for the preparation of EAC'4 with guinea pig or with human complement. J Immunol. 1967 Aug;99(2):263–268. [PubMed] [Google Scholar]
- Brisson A. D., Scandella C. J., Bienvenüe A., Devaux P. F., Cohen J. B., Changeux J. P. Interaction of a spin-labeled long chain acylcholine with the cholinergic receptor protein in its membrane environment. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1087–1091. doi: 10.1073/pnas.72.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Formation of a hemolytically active cellular intermediate by the interaction between properdin factors B and D and the activated third component of complement. J Exp Med. 1973 Dec 1;138(6):1305–1313. doi: 10.1084/jem.138.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni E. B., Dalmasso A. P. The indiction by complement of a change in KSCN-dissociable red cell membrane lipids. J Immunol. 1976 Apr;116(4):1163–1169. [PubMed] [Google Scholar]
- Hadding U., Müller-Eberhard H. J. The ninth component of human complement: isolation, description and mode of action. Immunology. 1969 Jun;16(6):719–735. [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Nicholson A., Mayer M. M. On the mechanism of cytolysis by complement: evidence on insertion of C5b and C7 subunits of the C5b,6,7 complex into phospholipid bilayers of erythrocyte membranes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5076–5080. doi: 10.1073/pnas.72.12.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H., Capaldi R. A., Vanderkooi G. Evidence for boundary lipid in membranes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):480–484. doi: 10.1073/pnas.70.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Haxby J. A., Arroyave C. M., Müller-Eberhard H. J. Molecular analysis of the membrane attack mechanism of complement. J Exp Med. 1972 Mar 1;135(3):549–566. doi: 10.1084/jem.135.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kury P. G., Ramwell P. W., McConnell H. M. The effect of prostaglandins E1 and E2 on the human erythrocyte as monitored by spin labels. Biochem Biophys Res Commun. 1974 Jan 23;56(2):478–483. doi: 10.1016/0006-291x(74)90867-5. [DOI] [PubMed] [Google Scholar]
- Longmuir K. J., Capaldi R. A., Dahlquist F. W. Nuclear magnetic resonance studies of lipid-protein interactions. A model of the dynamics and energetics of phosphatidylcholine bilayers that contain cytochrome c oxidase. Biochemistry. 1977 Dec 27;16(26):5746–5755. doi: 10.1021/bi00645a015. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Giavedoni E. B., Dalmasso A. P. Complement-induced decrease in membrane mobility: introducing a more sensitive index of spin-label motion. Biochemistry. 1977 Mar 22;16(6):1196–1201. doi: 10.1021/bi00625a026. [DOI] [PubMed] [Google Scholar]
- Michaels D. W., Abramovitz A. S., Hammer C. H., Mayer M. M. Increased ion permeability of planar lipid bilayer membranes after treatment with the C5b-9 cytolytic attack mechanism of complement. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2852–2856. doi: 10.1073/pnas.73.8.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Ohnishi S., Kitamura H., Inai S. Membrane fluidity change in erythrocytes induced by complement system. Biochemistry. 1976 Nov 2;15(22):4838–4843. doi: 10.1021/bi00667a013. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Onishi S. Organization of lipids in sarcoplasmic reticulum membrane and Ca2+-dependent ATPase activity. J Biochem. 1975 Nov;78(5):1039–1045. doi: 10.1093/oxfordjournals.jbchem.a130981. [DOI] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Ohnishi S. I. A spin-label study of biological membranes with special emphasis on calcium-induced lateral phase separation. Adv Biophys. 1976;8:35–82. [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. Purification of the sixth and seventh component of human complement without loss of hemolytic activity. J Immunol. 1976 Feb;116(2):263–269. [PubMed] [Google Scholar]
- Shin M. L., Paznekas W. A., Abramovitz A. S., Mayer M. M. On the mechanism of membrane damage by C: exposure of hydrophobic sites on activated C proteins. J Immunol. 1977 Oct;119(4):1358–1364. [PubMed] [Google Scholar]
- Stier A., Sackmann E. Spin labels as enzyme substrates. Heterogeneous lipid distribution in liver microsomal membranes. Biochim Biophys Acta. 1973 Jul 6;311(3):400–408. doi: 10.1016/0005-2736(73)90320-9. [DOI] [PubMed] [Google Scholar]
- Stolfi R. L. Immune lytic transformation: a state of irreversible damage generated as a result of the reaction of the eighth component in the guinea pig complement system. J Immunol. 1968 Jan;100(1):46–54. [PubMed] [Google Scholar]
- Tamura N., Shimada A., Chang S. Further evidence for immune cytolysis by antibody and the first eight components of complement in the absence of C9. Immunology. 1972 Jan;22(1):131–140. [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973 May 25;307(3):491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F., Smith I. C. The action of melittin on phosphatide multibilayers as studied by infrared dichroism and spin labeling. A model approach to lipid-protein interactions. Biochim Biophys Acta. 1974 Apr 12;345(1):129–140. doi: 10.1016/0005-2736(74)90252-1. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reconstitution of a calcium pump using defined membrane components. Proc Natl Acad Sci U S A. 1974 Mar;71(3):622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Bell R. M. Membrane matrix disruption by melittin. Biochim Biophys Acta. 1972 Nov 2;288(2):255–262. doi: 10.1016/0005-2736(72)90246-5. [DOI] [PubMed] [Google Scholar]


