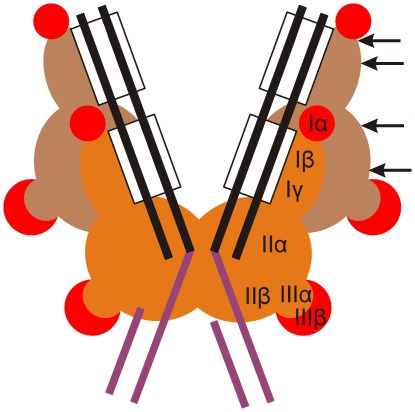
Figure 8. Mu transpososome architecture at post-integration stage and MuA regions allowing pentapeptide insertions.
The overall organization is sketched according to the unpublished crystallographic structure of Mu transpososome (P. Rice and S.P. Montaño, personal communication). The structure constitutes an essential framework for a meaningful interpretation of the functional data (see Discussion). Transposon end segments are shown with black lines, MuA binding sites (R1 and R2) are highlighted with rectangles, and the attached target DNA is shown in magenta. The catalytic MuA protomers are shown in orange and the non-catalytic protomers in brown. Insertion-tolerant subdomains are highlighted with red. The arrows (shown only for one protomer) indicate those linker and loop regions, in which insertions are tolerated well (wild type protein activity retained). The catalytic protomers act in trans, i.e. the protomer bound to one end catalyzes DNA cleavage and joining reactions in the other end.