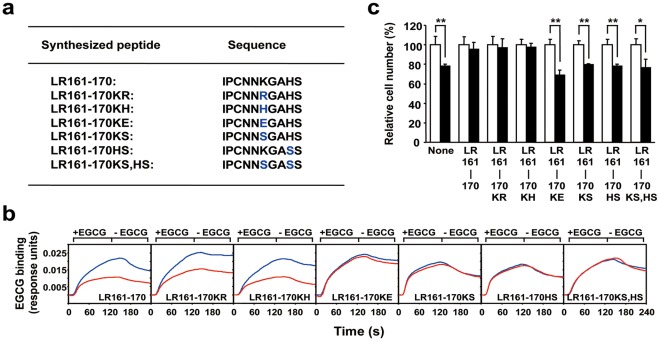
Figure 4. Importance of basic amino acid resides in the EGCG sensing motif for EGCG’s activity.
A) Basic amino acid replacements of LR161-170. Replaced peptide sequences are shown in the list. B) The neutralizing activity of several basic amino acid-replaced LR161-170 segments for the cell-surface binding of EGCG. After incubation of EGCG with each peptide at a molar ratio of 1∶1 in PBS, interactions between these EGCG-peptide mixtures and the cells were measured by a SPR assay. Sensorgrams of net binding of EGCG, which is the value of the subtracted peptide-binding signal from the total mixture-binding signal, are shown. The results are represented as EGCG alone (blue line) and EGCG plus deletion mutant of LR161-170 (red line). C) The neutralizing activity of several basic amino acid-replaced LR161-170 segments on the EGCG-induced inhibition of cancer cell growth. After incubation of EGCG with each peptide, the 67LR-overexpressed HepG2 cells were treated with the mixtures for 5 days and the cell number was assessed. The results, EGCG plus peptide (closed bar), are shown as the relative cell number to the EGCG-nontreated control (open bar), and the data presented are the means ± S.D. (n = 3) (Student’s t-test, *, p<0.05, **, p<0.01).