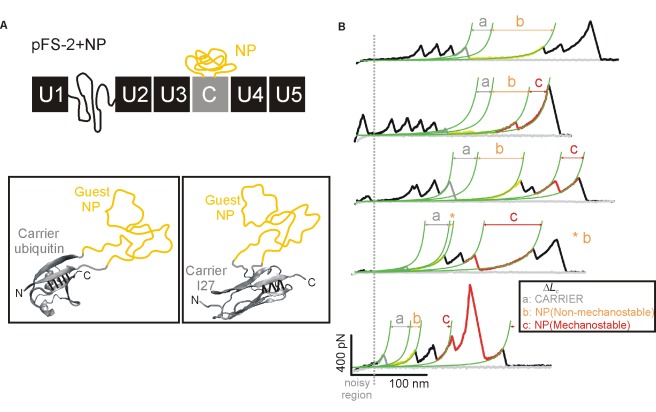
Figure 1. Nanomechanical analysis of NPs using the pFS-2 strategy.
(A) Top: Schematic representation of the pFS-2 polyprotein [30] carrying a guest NP (in orange) mechanically protected within a carrier module (C, in gray), flanked by ubiquitin repeats (U, in black). Bottom: cartoon representations of the two carrier-guest constructions used in this study: ubiquitin (left, PDB code 1d3z) and titin I27 (right, PDB code 1tit). The images were prepared by VMD 1.8.6 [58]. The hydrogen bonds of the mechanical clamp in both carriers are indicated by black bars [51]. (B) Representative force-extension recordings of pFS-2+Sup35NM. Using this strategy, we can unambiguously resolve a variety of conformations adopted by Sup35NM, ranging from a typical NM conformation (top trace, in orange), to M conformations with different degrees of mechanical stability (in red), including hM conformers (bottom). This color code will be maintained throughout the rest of the article. On the basis of our carrier-guest design, the carrier module must always unfold completely (“a” is ΔL c for the carrier module) before the force can access and stretch the guest NP, resulting in its unfolding (“b” and “c” represent the ΔL c for NM and M regions of the NP, respectively). The sum of b+c corresponds to the complete unfolding of the NP. The pFS-2 vector also contains an NM region, represented as a coil (a fragment of titin N2B [30]) that can act as a spacer to avoid the noisy proximal region of the force-extension recordings, a major problem in SMFS.