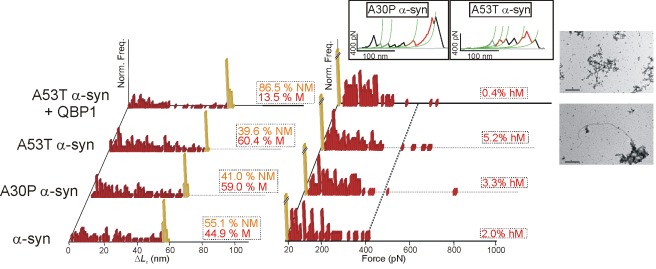
Figure 4. Nanomechanical analysis of α-synuclein.
ΔL c (left) and F (right) histograms of pFS-2 polyproteins carrying α-synuclein. The wt protein (first row) exhibits a wide-range polymorphism ranging from NM conformers (orange bars) to M conformers (red bars), including some hM conformers. Familial-disease mutations A30P and A53T increase the number of M and hM conformers of α-synuclein when compared to the wt. Treatment with QBP1 peptide (20 µM [42]) reduces the formation of M and hM conformers in A53T α-synuclein. TEM images of the amyloid fibers formed by ubi+A53T α-synuclein are shown on the right in which amyloid fibers are clearly not formed in the presence of QBP1 (top image). From bottom to top, the scale bars correspond to 0.45 and 0.6 µm, respectively. Examples of hM conformers of A30P and A53T α-synuclein are shown in the inset.