Abstract
MicroRNAs (miRNAs) are a class of endogenous, small, non-coding RNAs that regulate gene expression by targeting mRNAs and inhibiting expression via translation repression or RNA degradation. Emerging evidence indicates that miRNAs play a crucial role in the pathogenesis of human diseases, including tumor development. We profiled the miRNA expression between mature ovarian teratoma samples and matched normal tissues using miRNA microarrays, followed by validation with quantitative RT-PCR (qRT-PCR). The most highly expressed miRNAs in mature ovarian teratoma tissues were miRNA-520a-5p, miRNA-26b*, miRNA-421, miRNA-492 and miRNA-555, with a 1.3- to 2.6-fold change, whereas the least expressed miRNAs were miRNA-142-3p, let-7a, miRNA-19a, miRNA-34a, miRNA-620, miRNA-934, miRNA-657, miRNA-720, miRNA-22, miRNA-629 and miRNA-214, with a decreased level of 55–87% compared with normal tissues. The findings of the present study are the first to provide an altered miRNA profile for mature ovarian teratomas and differentially expressed miRNAs, which, if validated in future studies, may be essential in the pathogenesis of mature ovarian teratomas.
Keywords: microRNAs, mature ovarian teratomas, microarrays
Introduction
Mature cystic teratomas of the ovary are one of the most common benign ovarian neoplasms, accounting for approximately 10–20% of all ovarian tumors (1). Teratoma may occur at any age in women, but predominantly occurs in younger patients (20–40 years old) (2,3). Common complications for mature cystic teratomas include torsion, rupture, malignant transformation, infection and autoimmune hemolytic anemia (4,5), the prevalence of which is approximately 20%. The exact molecular mechanism underlying the formation of mature cystic teratomas, however, remains to be determined.
microRNAs (miRNAs) are a class of approximately 20–22 nucleotide small non-coding RNAs that regulate gene expression by binding to the 3′ untranslated regions (UTRs) of their target mRNAs and inhibiting the translation and/or promoting the degradation of mRNAs (6,7). miRNAs not only play significant roles in various biological processes but are also involved in pathological processes (8,9). Mounting evidence has shown that an abnormal expression of miRNAs is involved in human cancer, including ovarian tumors. In their study, Corney et al revealed that a reduced expression of miR-34b*/c may be particularly significant for progression to the most advanced stages and that the miR-34 family plays a crucial role in epithelial ovarian cancer pathogenesis (10). miRNA-15a and miRNA-16 target the Bmi-1 3′ UTR and significantly correlate with Bmi-1 protein levels in ovarian cancer and cell lines (11). However, the role of miRNAs in mature ovarian teratomas has yet to be elucidated.
The aim of the present study was to analyze miRNA differential expression profiles for mature ovarian teratoma tissues compared with normal tissues. Our data demonstrate that certain miRNAs may be relevant to the development of mature ovarian teratomas.
Materials and methods
Patients and methods
Three patients with mature cystic teratomas were recruited at the Department of Gynecology, Nanjing Maternal and Child Health Hospital of Nanjing Medical University (China) between March and August 2010. This study was approved by the institute’s ethics committee and informed consent was provided by each patient. The patients were pathologically confirmed as having mature cystic teratoma. Teratoma samples were obtained from the growth nidus of the cyst or from the innermost cell wall, but excluded any material from the ovarian capsule. The samples were washed with a sufficient amount of cold saline to reduce blood contamination (12). Tissues were immediately removed and preserved in liquid nitrogen and stored at −80°C until they were processed.
Total RNA preparation
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA quality and quantity was measured using a nanodrop spectrophotometer (ND-1000, Nanodrop Technologies; Wilmington, DE, USA) and RNA integrity was determined by gel analysis.
miRNA microarray
Microarrays were performed by utilizing the miRCURY Locked Nucleic Acid (LNA) microarray platform (Exiqon, Vedbaek, Denmark). All procedures were carried out according to the manufacturer’s instructions. Briefly, purified RNA was labeled with a miRCURY™ Hy3™/Hy5™ Power labeling kit (Exiqon). After discontinuing the labeling procedure, the Hy3™-labeled samples and Hy5™-labeled reference pool RNA samples were then mixed pair-wise and hybridized on the miRCURY™ LNA array (version 14.0) (Exiqon). The hybridization and slide washing were performed according to the miRCURY LNA array manual. The results were subjected to unsupervised hierarchical clustering (cluster 3.0) and TreeView analysis (Stanford University, Standford, CA, USA). Data were normalized using the locally weighted scatter plot smoothing (lowess) regression algorithm (MIDAS, TIGR Microarray Data Analysis System). Following scale normalization, replicated miRNAs were averaged. Differentially-expressed miRNAs, with statistical significance, were identified through volcano plot filtering. The results of hierarchical clustering were performed by MEV software (v4.6, TIGR).
Quantitative RT-PCR of miRNA
The total RNA from tissue was extracted with TRIzol reagent (Invitrogen) following the manufacturer’s instructions. RNA samples were quantified spectrophotometrically at 260 nm, and RNA integrity was verified by agarose-formaldehyde gel electrophoresis. In the reverse transcription step, complementary DNA (cDNA) was reversely transcribed from total RNA samples with a miRNA-specific stem-loop primer using the TaqMan microRNA reverse transcription kit (ABI, Forest City, CA, USA). Reverse transcription was carried out in reaction mixtures containing 1 μg total RNA, 0.5 μl miRNA reverse primer (1 μM), 0.3 μl RNase inhibitor (40 U/μl), 2 μl 10X buffer, 2 μl RNasin (10 U/μl) and an appropriate amount of RNAse-free H2O to a total volume of 20 μl. The reaction was performed at 16°C for 30 min and at 42°C for 40 min, followed by heat inactivation at 85°C for 5 min. Subsequently, quantitative real-time PCR was performed using ABI 7300 real-time PCR system (Applied Biosystems, CA, USA). The 25 μl PCR reaction included 2.5 μl RT product, 0.5 μl forward and reverse primer and 12.5 μl SYBR-Green real-time PCR master. The reactions were incubated in a 96-well optical plate at 95°C for 10 min, followed by 40 cycles of 95°C for 15 min and 60°C for 1 min. Reactions were run at least in triplicate. miRNA probe sets were specifically selected from reported miRNA studies in human blastocysts (13) or human tumor-associated tissue (14,15). The U6 expression level was used as an internal control for miRNA expression levels.
miRNA target predictions
miRNA target genes were predicted by miRGen Targets (http://www.diana.pcbi.upenn.edu/cgi-bin/miRGen/v3/Targets.cgi).
Statistical analysis
Data were analyzed by the Student’s t-test using the SPSS 15.0 statistical package (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to be statistically significant.
Results
miRNA expression profiles in mature ovarian teratoma relative to normal tissues
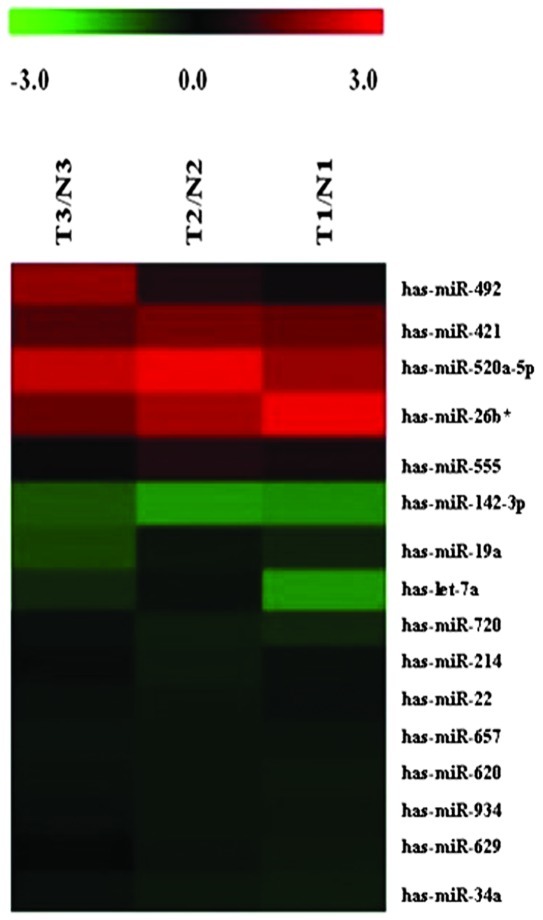
We detected 1,223 human mature miRNAs (miRbase v16.0) using miRNA microarrays and profiled the expression of miRNAs in mature ovarian teratoma. A total of 16 miRNA levels were significantly different between mature cystic teratomas and normal tissues (Fig. 1, Table I; P≤0.05). A total of 5 miRNAs were found to be over-expressed in mature ovarian teratoma tissues, with a 1.30- to 2.63-fold change; whereas 11 miRNAs were down-regulated, with a decreased level of 55–87% compared with the non-tumorous tissues.
Figure 1.
miRNAs differentially expressed in mature ovarian teratomas versus normal tissues. The rows show individual genes, while the columns show individual tissue samples. Red denotes a high expression and green denotes a low expression. T1-T3, tumor tissues; N1-N3, normal tissues; miRNA, microRNA.
Table I.
Relative expression of 16 miRNAs in mature ovarian teratomas and matched normal tissues.
| Gene name | Regulation | Fold-change | P-value |
|---|---|---|---|
| has-miRNA-520a-5p | Up | 2.6342413 | 0.002060 |
| has-miRNA-26b* | Up | 1.4913426 | 0.043586 |
| has-miRNA-421 | Up | 1.3811204 | 5.03E-06 |
| has-miRNA-492 | Up | 1.3156408 | 0.008240 |
| has-miRNA-555 | Up | 1.3002984 | 0.015700 |
| has-miRNA-142-3p | Down | 0.4544302 | 0.035932 |
| has-let-7a | Down | 0.4107124 | 0.007090 |
| has-miRNA-19a | Down | 0.3691387 | 0.049331 |
| has-miRNA-34a | Down | 0.3149833 | 6.25E-0.6 |
| has-miRNA-620 | Down | 0.2925045 | 0.036400 |
| has-miRNA-934 | Down | 0.2910072 | 0.041420 |
| has-miRNA-657 | Down | 0.2809156 | 0.005450 |
| has-miRNA-720 | Down | 0.2026972 | 0.033304 |
| has-miRNA-22 | Down | 0.1849261 | 0.002740 |
| has-miRNA-629 | Down | 0.1529428 | 0.014600 |
| has-miRNA-214 | Down | 0.1347023 | 0.007200 |
miRNA, microRNA.
Quantitative RT-PCR analysis
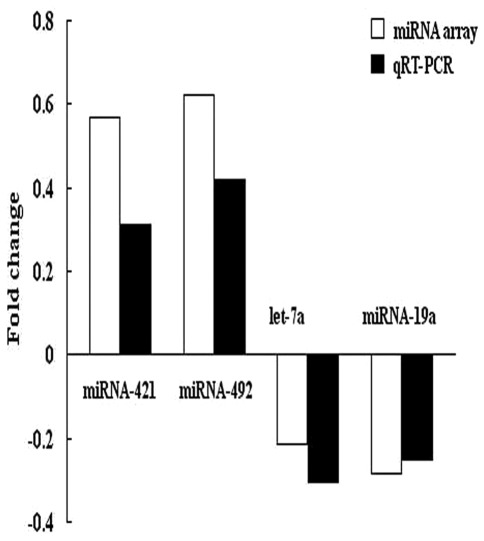
To confirm the results of microarray analysis, we performed quantitative RT-PCR analysis on the same number of samples using probes corresponding to miRNA-421, miRNA-492, let-7a and miRNA-19a. The expression data obtained by qRT-PCR analysis were comparable to the results observed in the microarray analysis (Fig. 2).
Figure 2.
Comparison of miRNA fold-changes by microarray and real-time qRT-PCR. Triplicate assays were performed from each RNA sample. Data were normalized using U6 as an endogenous control for RNA input. Fold-changes for these miRNAs from array and real-time qRT-PCR are shown as the mean. miRNA, microRNA; qRT-PCR, quantitative RT-PCR.
miRNA target prediction
To explore potential target genes of miRNAs, we used miRGen Targets (http://www.diana.pcbi.upenn.edu/cgi-bin/miRGen/v3/Targets.cgi). Among the miRNA whose expression was significantly different between tumor and non-tumorous samples, we found the marker gene for benign teratomas, HDAC1, which is the target gene of miRNA-142-3p and miRNA-34a.
Discussion
miRNAs play a crucial role in ovarian function (including folliculogenesis, oocyte maturation, ovulation, fertilization, cleavage, implantation and maintenance of pregnancy). Aberrant patterns of miRNA expression have also been reported in ovarian tumors (16–18). In the present study, we systematically analyzed the miRNA profile and identified significantly altered miRNAs in mature ovarian teratoma samples compared with normal tissues. A total of 5 up-regulated miRNAs and 11 down-regulated miRNAs were found in teratoma versus non-tumorous tissues. The microarray results were further confirmed via qRT-PCR for four selected miRNAs (miRNA-421, miRNA-492, let-7a and miRNA-19a).
DNA damage is a very common event in the life-cycle of a cell. DNA damage is also the underlying cause of specific mutations in cells leading to cancer, including ovarian cancer (19,20). Ataxia-telangiectasia mutated (ATM) is a serine/threonine kinase that plays a key role in the DNA damage response process (21). In their study, Hu et al found that human miR-421 suppresses ATM expression by targeting the 3′ UTR of ATM transcripts (15). In our tumor samples an up-regulation of miR-421 was observed, indicating that up-regulated miR-421 may affect DNA damage repair by suppressing ATM expression in ovarian teratoma tissues.
miR-421 has previously been described as up-regulated in late-stage ovarian carcinomas compared to early-stage cancer (22). In the present study, we found that miR-421 was also up-regulated in mature ovarian teratomas versus non-tumorous tissues. The results indicate that miR-421 expression is both up-regulated in benign and malignant tumors. Our study results further confirmed that miR-421 plays a key role during the initiation and development of ovarian carcinomas.
One of the findings among the lower expressed miRNAs are miRNA-142-3p and miRNA-34a. We obtained the same target gene, HDAC1 of miRNA-142-3p and miRNA-34a, by target prediction analysis. The finding indicates that miRNA-142-3p and miRNA-34a are capable of reducing the expression level of their target gene, HDAC1. HDAC1 is a member of the histone deacetylase (HDAC) family. Proteins of this family are critical cellular regulators that are involved in fundamental cellular events, including cell cycle progression, differentiation and tumorigenesis (23,24). HDAC1 was predominantly detected in mature areas of differentiated tumours (teratomas), rendering HDAC1 a potential novel biomarker for benign teratomas (25). In the present study, we demonstrated that miRNA-142-3p and miRNA-34a were lowly expressed in mature ovarian teratomas, meaning that HDAC1 had a relatively high expression in mature ovarian teratomas. Thus, these results are consistent with previous findings.
In conclusion, the present study was the first to demonstrate the differential profile of 16 miRNAs in mature ovarian teratomas. An aberrant expression of miRNAs may be essential for the pathogenesis of mature ovarian teratomas. Future studies addressing the function of these miRNAs are required to provide insights into their role in the development of ovarian teratomas.
Acknowledgements
This study was supported by grants from the Nanjing Science and Technology Agency (No. 200901084).
References
- 1.Matz MH. Benign cystic teratomas of the ovary: a review. Obstet Gynecol Surv. 1961;16:591–594. doi: 10.1097/00006254-196110000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Lakkis WG, Martin MC, Gelfand MM. Benign cystic teratoma of the ovary: a 6-year review. Can J Surg. 1985;28:444–446. [PubMed] [Google Scholar]
- 3.Ayhan A, Bukulmez O, Genc C, Karamursel BS, Ayhan A. Mature cystic teratomas of the ovary: case series from one institution over 34 years. Eur J Obstet Gynecol Reprod Biol. 2000;88:153–157. doi: 10.1016/s0301-2115(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 4.Emin U, Tayfun G, Cantekin I, Ozlem UB, Umit B, Leyla M. Tumor markers in mature cystic teratomas of the ovary. Arch Gynecol Obstet. 2009;279:145–147. doi: 10.1007/s00404-008-0688-2. [DOI] [PubMed] [Google Scholar]
- 5.Park SB, Kim JK, Kim KR, Cho KS. Imaging findings of complications and unusual manifestations of ovarian teratomas. Radiographics. 2008;28:969–983. doi: 10.1148/rg.284075069. [DOI] [PubMed] [Google Scholar]
- 6.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Shah AA, Meese E, Blin N. Profiling of regulatory microRNA transcriptomes in various biological processes: a review. J Appl Genet. 2010;51:501–507. doi: 10.1007/BF03208880. [DOI] [PubMed] [Google Scholar]
- 10.Corney DC, Hwang CI, Matoso A, Vogt M, Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH, Hermeking H, Nikitin AY. Frequent downregulation of miR-34 family in human ovarian cancers. Clin Cancer Res. 2010;16:1119–1128. doi: 10.1158/1078-0432.CCR-09-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya R, Nicoloso M, Arvizo R, Wang E, Cortez A, Rossi S, Calin GA, Mukherjee P. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res. 2009;69:9090–9095. doi: 10.1158/0008-5472.CAN-09-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miura K, Obama M, Yun K, Masuzaki H, Ikeda Y, Yoshimura S, Akashi T, Niikawa N, Ishimaru T, Jinno Y. Methylation imprinting of H19 and SNRPN genes in human benign ovarian teratomas. Am J Hum Genet. 1999;65:1359–1367. doi: 10.1086/302615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakshmipathy U, Love B, Goff LA, Jornsten R, Graichen R, Hart RP, Chesnut JD. MicroRNA expression pattern of undifferentiated and differentiated human embryonic stem cells. Stem Cells. 2007;16:1003–1016. doi: 10.1089/scd.2007.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Frowein J, Pagel P, Kappler R, von Schweinitz D, Roscher A, Schmid I. MicroRNA-492 is processed from the keratin 19 gene and up-regulated in metastatic hepatoblastoma. Hepatology. 2011;53:833–842. doi: 10.1002/hep.24125. [DOI] [PubMed] [Google Scholar]
- 15.Hu H, Du L, Nagabayashi G, Seeger RC, Gatti RA. ATM is down-regulated by N-Myc-regulated microRNA-421. Proc Natl Acad Sci USA. 2010;107:1506–1511. doi: 10.1073/pnas.0907763107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118:1944–1954. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaksman O, Stavnes HT, Kærn J, Trope CG, Davidson B, Reich R. miRNA profiling along tumor progression in ovarian carcinoma. J Cell Mol Med. 2010;15:1593–602. doi: 10.1111/j.1582-4934.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, D’Arcy T, McGuinness E, Sheils O, Sheppard B, O’Leary J. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35. doi: 10.1186/1476-4598-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Yu JJ, Mu C, Yunmbam MK, Slavsky D, Cross CL, Bostick-Bruton F, Reed E. Association between the level of ERCC-1 expression and the repair of cisplatin-induced DNA damage in human ovarian cancer cells. Anticancer Res. 2000;20:645–652. [PubMed] [Google Scholar]
- 20.Teodoridis JM, Hall J, Marsh S, Kannall HD, Smyth C, Curto J, Siddiqui N, Gabra H, McLeod HL, Strathdee G, Brown R. CpG island methylation of DNA damage response genes in advanced ovarian cancer. Cancer Res. 2005;65:8961–8967. doi: 10.1158/0008-5472.CAN-05-1187. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, III, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K, et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci USA. 2008;105:7004–7009. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. doi: 10.1111/j.1749-6632.2003.tb05964.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Rezai-Zadeh N, Seto E. Negative regulation of histone deacetylase 8 activity by cyclic AMP-dependent protein kinase A. Mol Cell Biol. 2004;24:765–773. doi: 10.1128/MCB.24.2.765-773.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simboeck E, Di Croce L. HDAC1, a novel marker for benign teratomas. EMBO J. 2010;29:3992–4007. doi: 10.1038/emboj.2010.281. [DOI] [PMC free article] [PubMed] [Google Scholar]