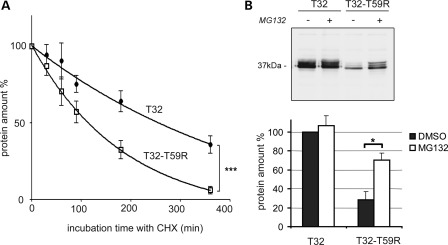
Figure 3.
The mutant protein Trisk 32-T59R is degraded faster, via the proteasome. (A) Trisk 32-T59R is degraded faster in the course of protein synthesis inhibition. WT Trisk 32 (T32) and mutant Trisk 32-T59R (T32-T59R) were first transfected in COS-7 cells. Transfected cells were then incubated with cycloheximine (CHX) to block protein synthesis for the indicated time. Cells were collected and analyzed by quantitative western blot with specific antibodies to evaluate the amount of protein. The curves show the quantification from four experiments of the amount of each protein (T32 -•- and T32-T59R -□-) compared with its initial amount. ***P < 0.001, F-test comparison of the two curves, based on decay and plateau parameters. (B) Inhibition of the proteasome raises the amount of the mutant protein Trisk 32-T59R. Twenty hours after transfection, the cells were incubated during 6 h with either dimethyl sulfoxide (DMSO) alone for controls (lanes ‘−’) or 50 µm MG132 to block proteasome (lanes ‘+’). The cells were then collected and analyzed by quantitative western blot with specific antibodies. The top panel shows a typical western blot, the bottom histogram (▪ DMSO; □ MG132) is a quantification (mean ± SEM) from three experiments. *P < 0.05, Mann and Whitney test comparison of DMSO- vs. MG132-treated cells.