Fig. 2.
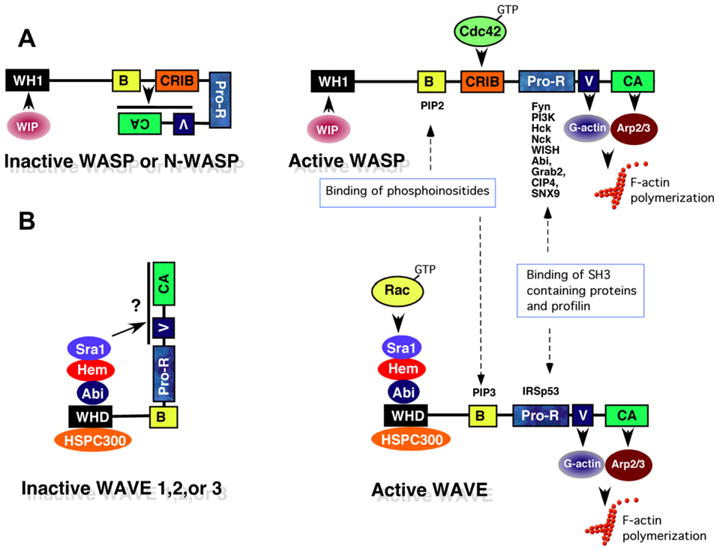
Activation of WASP and WAVE complexes by Rho-family GTPases. (A) Schematic diagram of the WASP complex. WASP [WASP or Neural WASP (N-WASP)] is basally inactive due to interaction of the basic region (B) and GTPase binding domains (CRIB) with the C-terminal verprolin homology/cofilin-homology/acidic region (VCA) domain, which prevents access to the Arp2/3 complex and G-actin. The interaction of Wip (WASP-interacting protein) with the WASP homology 1 domain (WH1) is believed to stabilize the inhibitory conformation. Following immunoreceptor activation, active Cdc42 (GTP-Cdc42) and PIP2 bind the CRIB and B domains respectively, thus releasing the VCA domain to bind G-actin and the Arp2/3 complex, which stimulates the branching of F-actin. In addition, the proline-rich region (Pro-R) of WASP acts as an adaptor protein by binding SH3-domain containing proteins (Fyn, PI3K, and Nck) which contribute to cellular activation, and Profilin which helps recruit actin monomers. (B) Schematic diagram of the WAVE complex. The WAVE complex is composed of pentameric subunits including Sra1, Hem (1 or 2), Abi (1 or 2), HSPC300 and WAVE (1, 2, or 3). Similar to WASP, the WAVE complex is also basally inactive, perhaps due to an interaction of the C-terminal VCA region with Sra1, which inhibits accessibility with G-actin and the Arp2/3 complex. Upon immunoreceptor stimulation, Rac-GTP binds to the WAVE complex via Sra1, which in the presence of PIP3 and IRSp53, may result in a structural change allowing the VCA region to bind G-actin and the Arp2/3 complex, resulting in F-actin polymerization. The binding of IRSp53 with the proline rich region of WAVE also enhances the ability to stimulate Arp2/3-driven actin polymerization, perhaps by recruiting WAVE to the plasma membrane [27,49].
