Fig. 4.
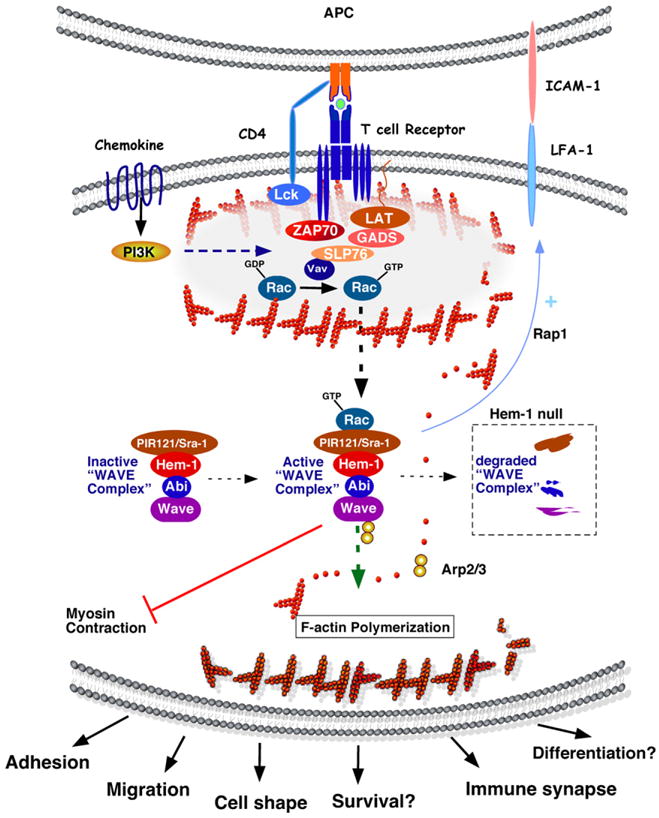
Model of Hem-1 functions in F-actin polymerization during T cell activation. T cell activation is induced by engagement of T cell receptor (TCR) complex on T cells with antigen peptide presented by major histocompatibility complexes (MHCs) on the surface of antigen presenting cells (APCs). Interaction of co-stimulatory molecules (CD28 and ICOS) on T cells with B7 family members on APCs (not shown) prevents anergy, thereby ensuring optimal activation. TCR-mediated signaling cascades are initiated by activation of the Src-family protein tyrosine kinase Lck which phosphorylates tyrosine residues in both CD3 complex and ZAP70. This results in recruitment of the adaptor proteins LAT, GADS, and SLP76, which are phosphorylated by ZAP70. LAT and SLP76 serve as scaffolds for adaptor molecules to regulate the F-actin polymerization via the Vav GEF, which activates Rac molecules. PI3K-mediated signaling facilitates cell survival, proliferation, and cytokine production in cooperation with TCR-mediated signaling. Interaction between LFA-1 and ICAM-1 is crucial for immune synapse formation. In immune cells, the WAVE complex [which consists of WAVE (1 or 2), Abi (1 or 2), Hem-1, Sra1, and HSPC300] is basally inactive. Upon T cell activation, activated Rac binds and activates the WAVE complex, thus promoting Arp2/3-mediated F-actin polymerization from monomeric G-actin. Hem-1 [also known as Nck-associated protein-1-like (NAP1L)] is a hematopoietic cell specific adaptor molecule which helps recruit and maintain a stable WAVE complex. Loss of Hem-1 (in Hem-1 null mice) destabilizes the interactions of WAVE complex subunits, resulting in WAVE protein degradation and impaired F-actin polymerization, which results in deficient adhesion (LFA-activation), immune synapse formation, migration, differentiation, and survival [10,83]. The WAVE complex (including Hem-1) may also directly control cell adhesion in a Rap1-dependent manner [47]. Hem-1 has also been implicated in inhibiting myosin contraction at the leading edge of migrating cells, which may contribute to membrane protrusion [43].
