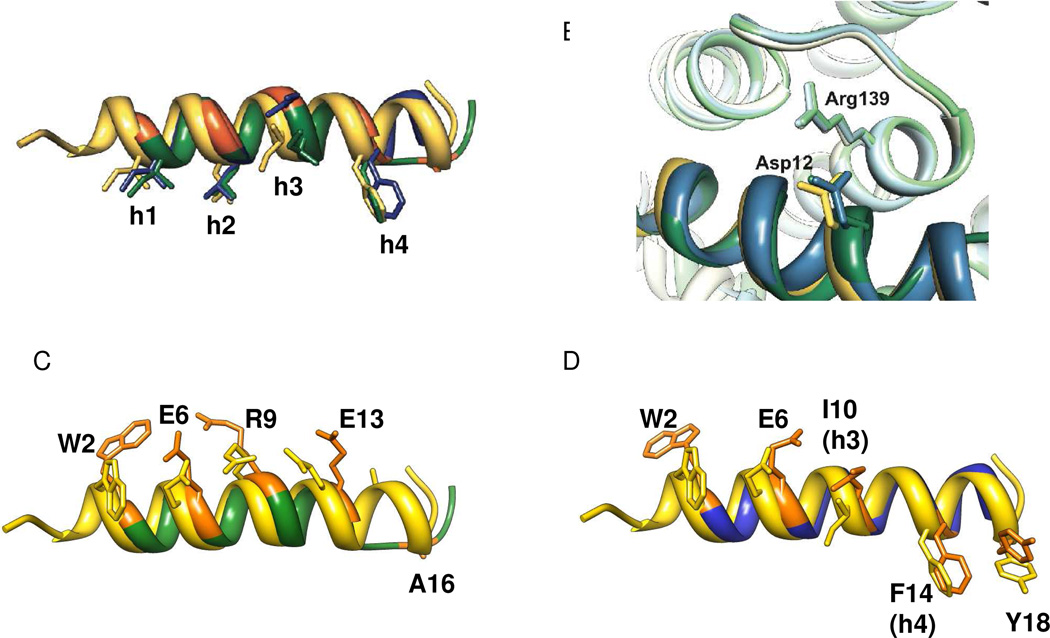
Figure 5.
Overlay of α-peptide 5 (yellow), α/β-peptide 2c (green), and α/β-peptide 4c (blue) from the co-crystal structures shown in Figure 4. Part A highlights the positions of the h1–h4 side-chains. Part B highlights the intermolecular salt bridge between an Asp side chain on the outward-facing side of the α- or α/β-peptide and the Arg139 side chain from the Bcl-xL. Parts C and D show pairwise comparisons of α/β-peptide 5 with either α/β-peptide 2c (C) or α/β-peptide 4c (D). These images compare the β3-residue side chains of 2c or 4c with the corresponding α-residue side chains from 5; these side chains sets display a consistent offset for 2c (C) but not for 4c (D) except for the Trp residue, as discussed in the text.