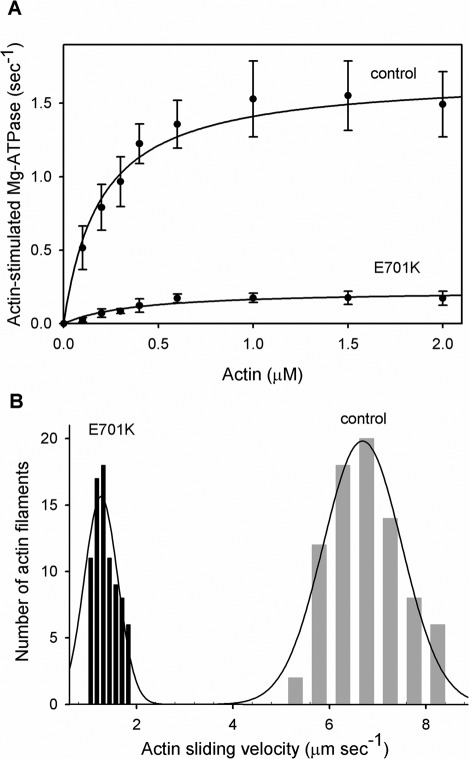
FIGURE 2:
Actin-stimulated MgATPase activity and actin sliding velocity of control (PwMhc2) and IBM-3 mutant (E701K) myosin. (A) ATPase activity of control and mutant myosin was determined in the absence and in the presence of F-actin. For the determination of Vmax and Km (Table 3), basal Mg-ATPase activities obtained in the absence of actin were subtracted from all data points. The data were fitted with the Michaelis–Menten equation to determine Vmax and Km (actin). The ratio of Vmax/Km from all assays was used to determine catalytic efficiency. CaATPase, MgATPase, actin-stimulated MgATPase activity, and the Vmax/Km ratio were significantly reduced (p < 0.001), whereas Km was significantly enhanced (p < 0.05) in mutant relative to control myosin (Table 3). (B) Actin sliding velocity stimulated by IBM-3 mutant (E701K) myosin was significantly reduced (p < 0.001) compared with that of control myosin. Our data suggest that the IBM-3 mutation depresses the enzymatic activity of the molecular motor.