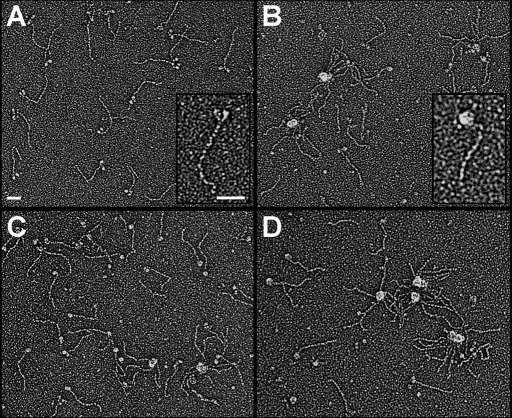
FIGURE 4:
The IBM-3 myosin mutation increases the molecular motor's structural lability. To directly determine whether the IBM-3 mutation alters the normal two-headed myosin structure exhibited by rotary shadowed wild-type molecules, we generated electron micrographs of PwMhc2 control and E701K myosin maintained at 23°C and after a 4-min incubation at 37°C. PwMhc2 myosin showed typical two-headed structures connected by a long, ∼150-nm tail segment (A). However, at the same temperature, E701K molecules often showed collapsed heads, and they frequently packed into aggregated clumps (B) reminiscent of wild-type motors exposed to elevated temperatures (C), where discrete S-1 heads of a given molecule were seldom resolved. Two-headed structures were rarely observed when either PwMhc2 or E701K myosin was briefly incubated at 37°C. (C, D). Insets show enlarged, individual PwMhc2 (A) or E701K (B) myosin at 23°C and clearly demonstrate intramolecular head aggregation of the mutant molecule. Bars, 50 nm.