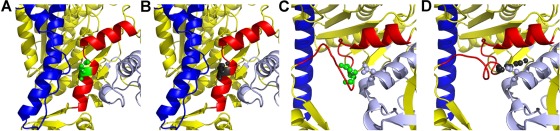
FIGURE 6:
Modeling of wild-type (Glu-706) and IBM-3 mutant (Lys-706) myosin (A) In the pre–power stroke state (PDB: 1QVI), Glu-706 (green) is part of the SH1-SH2 helix of myosin (red). For orientation the relay helix is shown in blue (B) Substitution of the IBM-3 mutation (Lys-706, gray spheres) does not have a major effect on the pre–power stroke structure (C) The wild-type SH1 helix containing Glu-706 (green spheres) melts during the power stroke, as shown here (red) in the post–power stroke state (PDB: 1KK8). (D) The IBM-3 mutation (Lys-706; gray spheres) is predicted to alter the orientation of the melted helix (red) in the post–power stroke state, bringing it in contact (2.8 Å) with Asp-98 (light blue spheres) in the N-terminal region. This new charge-based interaction may prevent reformation of the SH1 helical region of the SH1-SH2 helix after ATP binding.