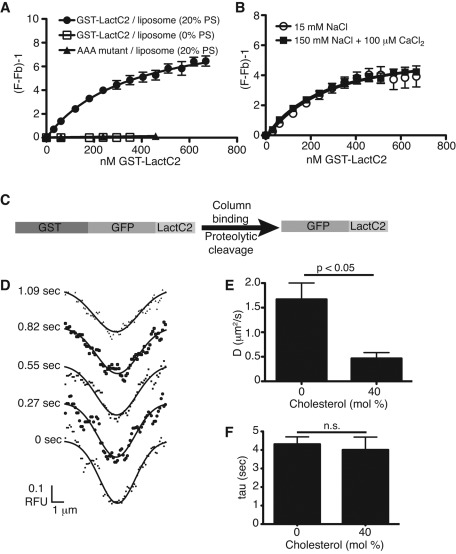
FIGURE 2:
PS-binding properties and bilayer dynamics of recombinant LactC2. (A) Binding isotherms of recombinant GST-LactC2 or GST-LactC2(AAA) to egg-PC/dansyl-PE (2.5 mol %) liposomes with or without 20% PS in 150 mM NaCl-containing buffer. The term (F/Fb) − 1 (y-axis) refers to the observed FRET fluorescence resulting from the close proximity of dansyl-PE in the liposome and tryptophan residues in GST-LactC2. The traces are nonlinear least-squares curves fit to a one-site binding hyperbola (Gilbert et al., 1990), yielding Kd for LactC2 PS binding of 351 ± 72 nM. (B) Effects of calcium and of changes in ionic strength on LactC2 binding to PS-containing liposomes. Ionic strength was reduced by lowering NaCl concentration to 15 mM. Where indicated, 100 μM CaCl2 was added. (C) Diagram of strategy for purification of GFP-LactC2. GST-GFP-LactC2 construct in pGEX-6p was expressed in E. coli, lysates were bound to glutathione–Sepharose, and GFP-LactC2 was cleaved on-column from GST with PreScission protease. (D) Sample Gaussian best fits of fluorescence intensity profiles obtained during FRAP of purified GFP-LactC2 bound to a supported lipid bilayer containing 15% DOPS (see Hammond et al., 2009, for details). (E, F) Diffusion coefficients (E) and τ (F) calculated for GFP-LactC2 association with supported lipid bilayers containing 15% DOPS and varying mol % cholesterol. Data are means ± SEM of 7–13 independent determinations from two experiments.