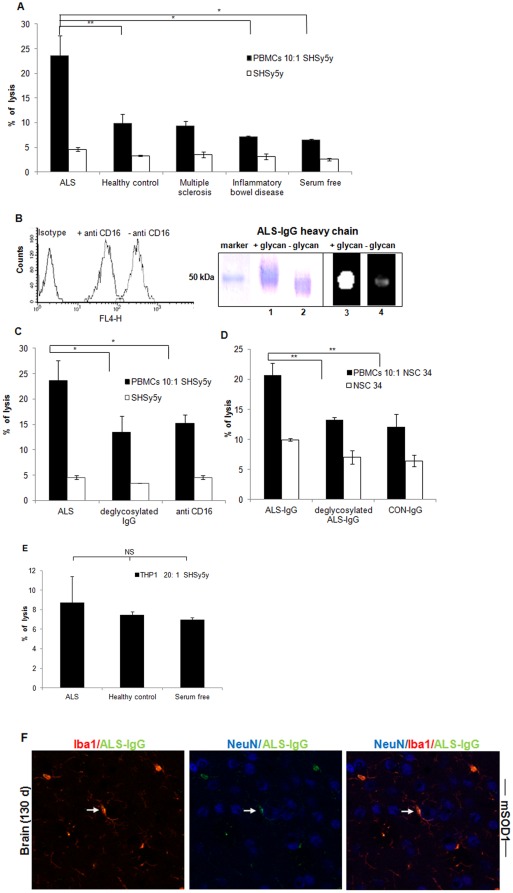
Figure 5. Killing human neuroblastoma or mouse NSC34 cells through the ADCC pathway.
ADCC was performed using human neuroblastoma as target cells, PBMCs as effector cells, and pools of serum samples of ALS, healthy control, inflammatory bowel disease patients, and multiple sclerosis patients as IgG sources. The controls contain: neuroblastoma cells incubated with IgG pools from the different serum sources and co-cultures of neuroblastoma cells and PBMCs (A). FACS results from PBMCs pre-treated with anti-CD16 antibodies (B, left) and the heavy chain of ALS-IgG before and after PNGase-F treatment in SDS-PAGE and Western blot using ECL lectin (B, right); ADCC mediated by CD16-blocked effector cells or by ALS-IgG after PNGase-F treatment, as compared to the ADCC against neuroblastoma mediated by unblocked effector cells and untreated ALS-IgG (C); Killing of NSC34 cells by ADCC as described above was mediated by intact (untreated) ALS-IgG, PNGase-F treated ALS-IgG and by IgG from healthy controls (D). Neuroblastoma lysis by CD32- and CD64-positive THP1 cells was mediated by ALS-IgG, IgG of healthy controls, and in serum free of IgG (E). Spontaneous lysis was measured in neuroblastoma or NSC34 cultures. Triple staining of NeuN, Iba1, and ALS-IgG by anti-human IgGs conjugated to FITC demonstrates the localization of intact ALS-IgG in immune synapse (arrow) amongst microglia and neurons (F). Data represent the mean ± SD of triplicate measurements from triplicate independent experiments. Pools of healthy and patient samples contained a mixture of four individual serum samples with similar glycan amounts represented in peaks 12 and 13. Statistical significance, ** p<0.01, * p<0.05 and NS (Not significant) is represented versus the appropriate controls in each panel.