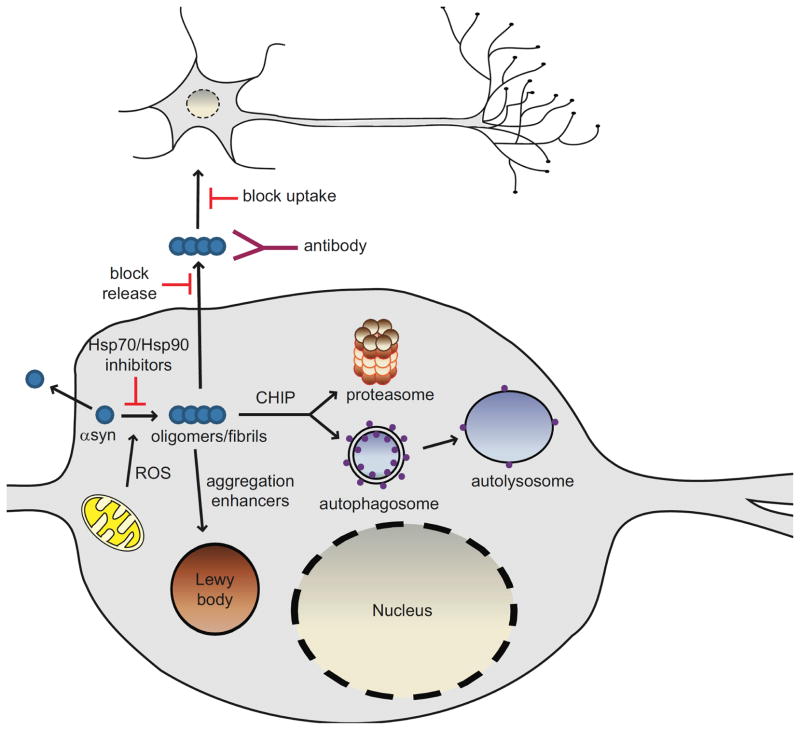
Fig. 1. Pathways of αsyn toxicity and potential therapeutic strategies.
Increasing evidence implicates soluble, oligomeric species of αsyn as the toxic genus in the pathogenesis of PD. Reactive oxygen species (ROS) can modify αsyn such that it has an increased tendency to aggregate. Interventions that target oligomeric forms of αsyn by inducing degradation, preventing aggregation, or increasing aggregation in favor of less toxic fibrillar species are all being considered as therapeutic strategies. Oligomerization of αsyn can be prevented by Hsp70 overexpression or Hsp90 inhibition. Lewy bodies are thought to be a mechanism by which the cell sequesters abnormal, toxic proteins and as such, aggregation enhancers may offer innovative approaches to reduce αsyn toxicity. The enhancement of clearance of αsyn aggregates via the stimulation of degradation pathways could help mitigate αsyn-induced dysfunction in these pathways. CHIP directs proteins towards degradation via both the proteasome and lysosome making CHIP an extremely attractive therapeutic target. αSyn may also be released from living cells into the surrounding extracellular milieu but the species released (monomer or oligomer) remains to be determined. Extracellular αsyn could act as a seed to promote aggregation of αsyn in neighboring cells, ultimately leading to αsyn toxicity or Lewy body formation. Blocking αsyn release and/or uptake by neighboring cells might halt the spread of αsyn pathology. Lowering the extracellular oligomeric burden by immunotherapy may also be an innovative and novel approach in the treatment of Parkinson’s disease.