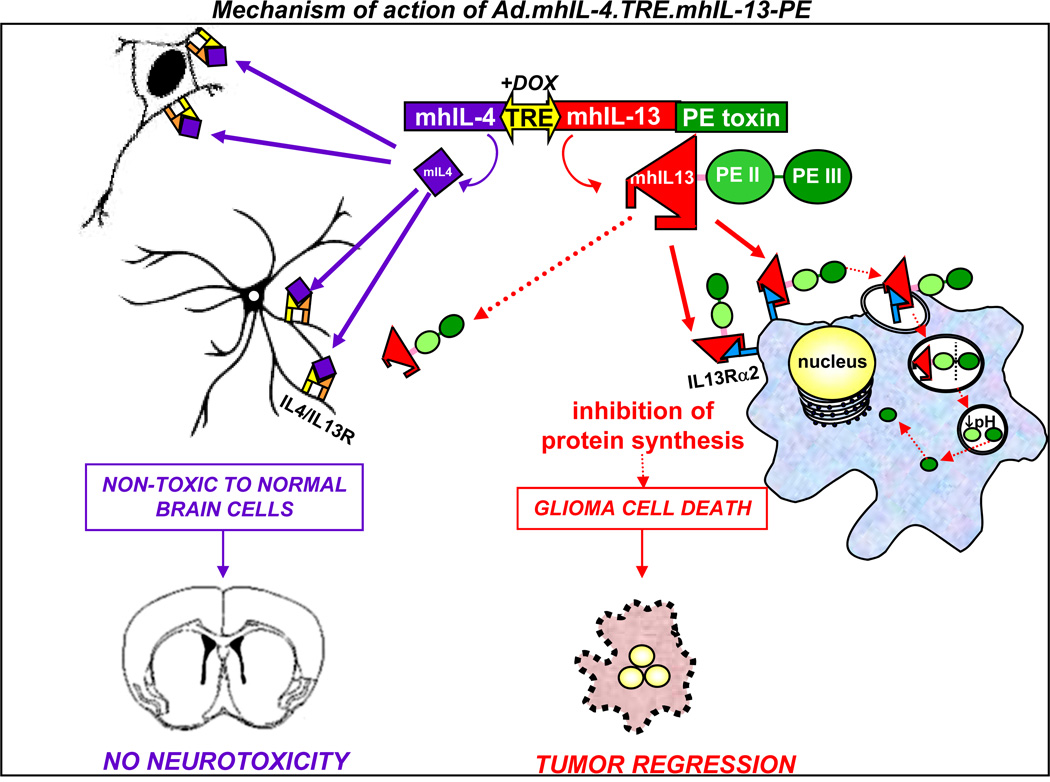
Figure 4. Gene therapy-mediated delivery of mhIL-13-PE leads to anti-tumor efficacy and long term survival in the absence of neurological toxicity.
The diagram depicts the mechanism of action of the targeted gene therapeutic strategy. The targeting of IL-13α2 receptor overexpressed in glioma cells has been approached by constructing an Ad vector encoding the truncated form of PE toxin fused to a mutated form of human IL-13 (mhIL-13), which has higher affinity for the glioma-associated IL-13α2 receptor and negligible binding to the physiological receptor, IL13/IL4R. Binding of mhIL-13-PE to IL13Rα2 promotes its internalization into glioma cells. Domain II (PEII) mediates the translocation of the toxin into the endosomes by endocytosis. Once in the endosomes, furin mediates proteolytic cleavage that activates the catalytic Domain III (PEIII). Due to the low pH of the endosome, the processed fragment of the toxin is translocated to the cytosol and inhibits protein synthesis, leading to glioma cell death. The absence of IL-13Rα2 protects normal cells from mhIL-13-PE mediated cell death. The safety of our approach has been further enhanced by co-expressing a mutated form of IL-4 (mhIL-4) encoded in the therapeutic bi-directional Ad which acts as an antagonist of the physiological receptor comprising the IL-13α1R and IL-4αR chains. Secreted IL-4 would inhibit the already negligible binding of mhIL-13-PE to normal brain cells, without affecting the binding of the mhIL-13-PE to GBM cells.