Abstract
Activation of NF-E2-related factor 2 (Nrf2) is a potential therapeutic intervention against endothelial cell oxidative stress and associated vascular disease. We hypothesized that treatment with the phytochemicals in the patented dietary supplement Protandim would induce Nrf2 nuclear localization and phase II antioxidant enzyme protein in human coronary artery endothelial cells (HCAECs), protecting against an oxidant challenge in an Nrf2- dependent manner. Protandim treatment induced Nrf2 nuclear localization, and HO-1 (778% of control ± 82.25 P < 0.01), SOD1 (125.9% of control ± 6.05 P < 0.01), NQO1 (126% of control ± 6.5 P < 0.01), and GR (119.5% of control ± 7.00 P < 0.05) protein expression in HCAEC. Treatment of HCAEC with H2O2 induced apoptosis in 34% of cells while pretreatment with Protandim resulted in only 6% apoptotic cells (P < 0.01). Nrf2 silencing significantly decreased the Protandim-induced increase in HO-1 protein (P < 0.01). Nrf2 silencing also significantly decreased the protection afforded by Protandim against H2O2- induced apoptosis (P < 0.01 compared to no RNA, and P < 0.05 compared to control RNA). These results show that Protandim induces Nrf2 nuclear localization and antioxidant enzyme expression, and protection of HCAEC from an oxidative challenge is Nrf2 dependent.
1. Introduction
Oxidative stress has been implicated in many chronic diseases including Alzheimer's, diabetes and coronary artery disease (CAD) [1–4]. Increased production of reactive oxygen species (ROS) and oxidative damage in the vascular endothelium contribute to CAD initiation and progression. Specifically, increased vascular superoxide causes oxidation of lipids, decreased nitric oxide availability, increased expression of adhesion molecules and inflammatory mediators, and recruitment of monocytes to the endothelium [5–8]. Endothelium-bound superoxide dismutase is also decreased in CAD patients compared to healthy controls, impairing the cellular response to excessive ROS production [9]. Atherosclerotic coronary arteries isolated from humans display increased superoxide production compared to nonatherosclerotic human coronary arteries, and in a mouse model of atherosclerosis, attenuation of superoxide production by decreased expression of NADPH oxidase (NOX) results in a decrease in atherosclerotic lesion size [10, 11].
Initial studies examining the effects of decreasing oxidative stress in several diseases, including cardiovascular disease, have used exogenous antioxidant supplements such as vitamins C and E. However, the protective effect of exogenous antioxidants has been disappointing and in some cases supplementation increased mortality [12–14]. A novel approach to decreasing disease-associated oxidative stress involves augmenting endogenous antioxidant defense systems rather than relying on exogenous antioxidant supplementation. Protandim is a commercially available dietary supplement consisting of phytochemicals derived from five widely studied medicinal plants including silymarin from milk thistle, curcumin from turmeric, bacopa extract, ashwagandha, and green tea extract. The five phytochemical components of Protandim have a synergistic effect to induce phase II antioxidant enzymes and protect cells from oxidative stress through activation of the transcription factor NF-E2-related factor 2 (Nrf2) [15, 16].
Nrf2 is constitutively expressed but is marked for ubiquitination by association with Kelch-like ECH-associated protein 1 (Keap1) in the cytosol. Activation of Nrf2 occurs when it is released from Keap1 and translocates to the nucleus. In the nucleus, Nrf2 heterodimerizes with small Maf or Jun proteins and binds to the antioxidant response element (ARE) in the promoter region of several hundred genes including many phase II antioxidant enzymes subsequently initiating transcription [17, 18]. Protandim likely activates Nrf2 through activation of various kinases with subsequent Nrf2 phosphorylation [16, 19].
Although acute activation of Nrf2 occurs in vivo in response to oxidized phospholipid signaling, increased ROS production, hyperglycemia, and shear stress [20–22], in chronic disease states the antioxidant response is often insufficient to maintain redox balance and prevent disease progression [22–24]. For example, Landmesser et al. report increased SOD activity in young hypercholesterolemic subjects compared to age-matched controls [9]. In contrast, decreased SOD activity was observed in coronary arteries from CAD patients compared to age-matched controls [9]. Data show that upregulation of phase II antioxidant enzymes can protect against oxidative stress in vitro and in humans [16, 25, 26]. It was also recently reported that Protandim protected a human saphenous vein ex vivo culture from oxidative stress-induced hyperplasia and vessel wall thickening [27]. Thus, phytochemical-induced Nrf2 activation is a potential therapeutic intervention against endothelial cell oxidative stress and associated vascular disease initiation and progression. Limited research (8 publications) exists examining whether Protandim treatment can minimize the pathologies associated with chronic diseases. The effects of Protandim on Nrf2 and oxidative stress in human coronary vascular cells have not been investigated.
The purpose of this study was to determine (1) if treatment with Protandim-induces Nrf2 nuclear translocation and phase II antioxidant enzyme protein expression in human coronary artery endothelial cells (HCAEC), (2) if treatment with Protandim protects HCAEC from apoptosis induced by an oxidant challenge, and (3) if Nrf2 mediates Protandim induced protection from an oxidative challenge. We hypothesized that Protandim treatment would induce Nrf2 nuclear localization and phase II antioxidant enzyme protein expression, and Protandim treatment prior to an oxidant challenge would afford cells protection in a Nrf2 dependent manner.
2. Materials and Methods
2.1. Materials
HCAEC and cell culture reagents, PrimeFect siRNA transfection reagent and PrimeFect diluent were purchased from Lonza (Walkersville, MD). Heme oxygenase-1 (HO-1) antibody was from Affinity Bioreagents (Golden, CO), nitroquinone oxidoreductase (NQO1), and glutathione reductase (GR) antibodies, and appropriate HRP-conjugated secondary antibodies were purchased from AbCam, (Cambridge, MA). Nrf2, Cu-Zn superoxide dismutase (SOD1), and actin antibodies, HRP- and FITC- conjugated appropriate secondary antibodies, and Nrf2 siRNA, and control RNA were purchased from Santa Cruz Biotech (Santa Cruz, CA). The Santa Cruz Nrf2 siRNA is a pool of 3 19–25 nt siRNA duplexes. Sequence one 5′ to 3′ sense: GCAUGCUACGUGAUGAGAtt, antisense: UCUUCAUCACGUAGCAUGCtt, sequence two 5′ to 3′ sense: CUCCUACUGUGAUGUGAAAtt, antisense: UUUCACAUCACAGUAGGAGtt, sequence three 5′ to 3′ sense: GUGUCAGUAUGUUGAAUCtt, antisense: UGAUUCAACAUACUGACACtt. The Pierce BCA assay kit for determining protein concentrations, protease and phosphatase inhibitors, and SuperSignal West Dura substrate were from Thermo Scientific (Rockford, IL). TUNEL assay kits were from Roche (Indianapolis, IN).
2.2. Culture of HCAEC
Primary HCAECs were grown in endothelial cell growth medium (EBM-2) containing 5% FBS and manufacturer-recommended supplemental growth factors, antibiotics, and antimycotics. All assays were performed on cells at 80–100% confluence, between passages 3 and 12, and were repeated at least 3 times in duplicate or triplicate.
2.3. Protandim and H2O2 Preparation and Treatment
Protandim is a phytochemical composition containing W. somnifera, B. monniera (45% bacosides), S. marianum, Ca. sinesis (98% polyphenols and 45% (−)-epigallocatechin-3-gallate), and curcumin (95%) from turmeric (Cu. longa) (LifeVantage Corp., Salt Lake City, UT). Protandim extract was prepared by mixing 500 mg Protandim in 5 mL 95% ethanol. The mixture was rocked overnight at room temperature and then centrifuged for 15 min at 3,000 ×g. The resulting supernatant contains ethanol extracted Protandim at a concentration of 100 mg/mL. The Protandim extract was diluted to 20 μg/mL with complete cell culture medium for all treatments following initial concentration response experiments that showed 20 μg/mL was the lowest concentration that significantly stimulated phase II antioxidant enzyme protein expression. Cells not treated with Protandim were treated with 95% ethanol as a vehicle control, with a maximum ethanol concentration in the growth medium of 0.02% (2 μL in 10 mL). H2O2 (30% W/W) was diluted in complete cell growth medium to a final concentration of 1.25 μM for all treatments. Protandim treatments ranged from 1 hr to 12 hrs as indicated, and H2O2 treatments were 4 hrs.
2.4. Western Blot Analyses
HCAEC were seeded in 65 mm polystyrene cell culture dishes and grown to at least 80% confluence prior to Protandim treatment. Following treatment, cells were scraped in RIPA buffer (50 mM Tris, 0.15 M NaCl, 1% Na deoxycholic acid, 1 mM EGTA, 1% NP40) containing protease and phosphatase inhibitors and sonicated 3 × 10 secs. Protein concentrations were determined using a BCA assay, and samples were diluted with Laemmli sample buffer. Samples were separated on 10% polyacrylamide gels at 125 v and transferred to nitrocellulose membranes (BioRad, Hercules, CA) for 1 hr at 50 v. Membranes were blocked for 1 hr in Superblock (Thermo Scientific, Rockford, IL) then incubated with primary antibodies against HO-1 (1 : 500), SOD1 (1 : 500), NQO1 (1 : 1000), GR (1 : 5000), and β-actin (1 : 1000) followed by the appropriate HRP-conjugated secondary antibodies. Membranes were developed by chemiluminescence using SuperSignal West Dura substrate (Thermo Scientific, Rockford, IL), with digital images obtained using the Biospectrum UVP system. All signals were normalized to β-actin obtained from the same blot and expressed as the percent of the vehicle control (no Protandim) condition.
2.5. Immunocytochemistry for Nrf2 Nuclear Localization
HCAECs were grown to confluence on coverslips in 35 mm polystyrene cell culture plates coated with 2 μg/cm2 fibronectin prior to Protandim treatment. Cells were initially treated with Protandim for 1 hr, 2 hrs, 4 hrs, 8 hrs, and 12 hrs to determine the optimal duration of treatment for visualizing Nrf2 nuclear localization. Following time course experiments, all Protandim treatments were for 1 hr. Cells were washed with PBS, fixed for 30 min in 4% paraformaldehyde, washed with PBS, and then permeabilized in cold acetone for 30 min. Cells were blocked for 1 hr in 5% bovine serum albumin with 0.5% goat serum and then incubated with Nrf2 primary antibody (1 : 100) for 1 hr at room temperature. Cells were washed with PBS then incubated for 45 min in FITC-conjugated secondary antibody at room temperature in the dark. The coverslips were mounted on slides using DAPI containing mounting medium for identification of cell nuclei and visualized by fluorescence microscopy (Nikon TE2000) using Metamorph data acquisition software (Molecular Devices, Sunnyvale, CA).
2.6. TUNEL Assay to Assess Apoptosis
A TdT-mediated dUTP nick end labeling (TUNEL) assay was used to assess HCAEC apoptosis in response to an oxidative challenge. Cells were grown to confluence on fibronectin-coated coverslips prior to Protandim and H2O2 treatment. Cells were washed with PBS, fixed for 30 min in 4% paraformaldehyde, washed again with PBS, then permeabilized for 2 min in 0.1% Triton X-100 with 0.1% sodium citrate. Cells were washed again with PBS and incubated in TUNEL reagent for 1.5 hrs. The coverslips were then mounted on slides using DAPI containing mounting medium to identify cell nuclei. Signals were visualized using fluorescence microscopy (Nikon TE2000).
2.7. Nrf2 Silencing
Prior to transfection, 250 μL PrimeFect diluent was mixed with 5 μL PrimeFect transfection reagent and incubated at room temperature for 15 min. Nrf2 siRNA or control RNA was added to the transfection solution for a final concentration of 50 nM and incubated at room temperature for 15 min. The transfection solution was applied to cells grown to 70–80% confluence in antibiotic free medium, along with 1.25 mL antibiotic free growth medium. The volume of transfection reagent used caused minimal distress to the cells as assessed by minimal changes to cell morphology. After 24 hrs, the transfection solution was removed and the cells were rinsed with PBS, treated with Protandim, and assayed as indicated.
2.8. Statistical Analysis
Unpaired t-tests were used to compare control versus Protandim treatments. A two by three treatment (Protandim and no Protandim) by condition (no RNA, control RNA, and Nrf2 siRNA) ANOVA with a priori linear contrasts of means was used to analyze Nrf2 silencing experiments. Percent protection was analyzed using one-way ANOVA. Statistical significance was set at P < 0.05.
3. Results
3.1. Antioxidant Enzyme Protein Expression Is Elevated in Response to Protandim Treatment
HCAEC were treated with Protandim concentrations of 0 to 50 μg/mL in 5 μg/mL increments to determine a profile of Nrf2 activation as measured by HO-1 protein content. HO-1 protein went from barely detectable in control conditions to 8–10-fold greater in cells treated with 20–30 μg/mL (Figure 1(a)). Concentrations higher than 30 μg/mL induced morphological changes. In all subsequent treatments 20 μg/mL Protandim was used. HO-1 protein was visible after 1 hr of Protandim treatment and became significant and sustained from 4 hrs through the longest treatment period of 12 hrs (data not shown). To confirm treatment concentration and duration on multiple antioxidant enzymes we determined that 20 μg/mL Protandim for 12 hrs induced HO-1 (778% of control + 82.25 P < 0.01), SOD1 (125.9% of control + 6.05 P < 0.01), NQO1 (126% of control + 6.5 P < 0.01), and GR (119.5% of control + 7.00 P < 0.05) (Figure 1(b)). All subsequent treatments used Protandim at a concentration of 20 μg/mL for 12 hrs unless otherwise noted.
Figure 1.
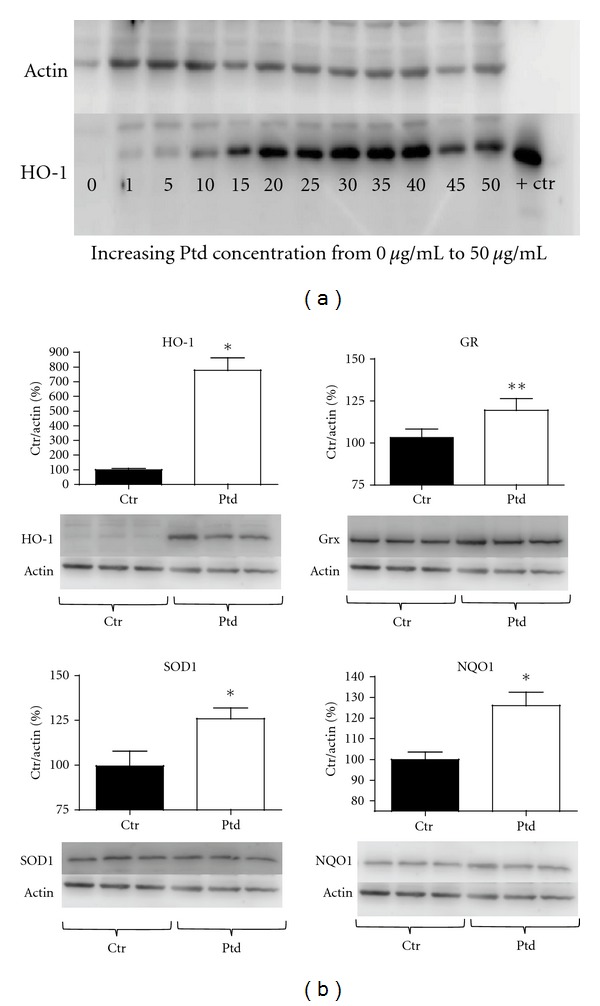
Protandim treatment induces phase II antioxidant enzyme protein expression in HCAEC. (a) HCAECs were treated with 1–50 μg/mL of Protandim for 12 hrs prior to measuring HO-1 protein expression. The HO-1 signal was verified using a purified HO-1 protein extract positive control. (b) Following determination of the appropriate concentration of Protandim, HCAECs were treated with 20 μg/mL Protandim for 12 hrs then protein expression of HO-1, GR, SOD-1, and NQO1 was determined by Western blot. All bands were normalized to β-actin as a loading control and expressed as a percent of the control (vehicle) condition (n = 16 treatments). *P < 0.01 compared to control (no Protandim), **P < 0.05 compared to control (no Protandim). Data are presented as mean + SE. Ptd: Protandim, Ctr: No Protandim, + Ctr: HO-1 positive control.
3.2. Protandim Stimulates Nrf2 Nuclear Localization
HCAECs were treated with Protandim for up to 12 hrs and subsequently visualized using immunocytochemistry to determine Nrf2 nuclear localization. Nrf2 content and nuclear localization were elevated as soon as 1 hr after Protandim treatment initiation. Induction remained with treatments of 2, 4, 8, and 12 hrs (12 hrs was the longest treatment duration examined) (Figure 2).
Figure 2.
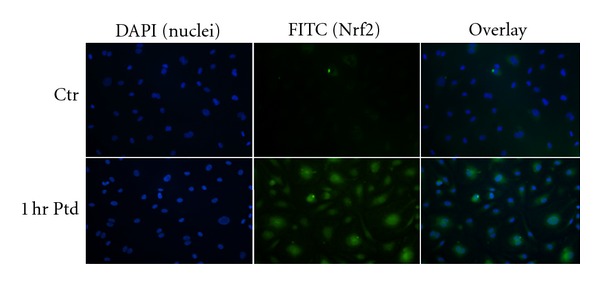
Protandim induced Nrf2 expression and nuclear localization. HCAECs were treated with Protandim for 1 hr, following which immunofluorescence was used to visualize changes in Nrf2 expression and localization. Following Protandim treatment Nrf2 signal was greater and became visible in the nucleus. Ptd: Protandim, Ctr: no Protandim.
3.3. Protandim Protects HCAEC from Oxidative Challenge Induced Apoptosis
HCAECs were treated for 12 hrs with Protandim or vehicle control followed by a 4 hr exposure to 1.25 μM H2O2 and induction of apoptosis was measured by TUNEL assay. Protandim was removed prior to H2O2, exposure. In vehicle controls apoptosis was induced in 34% of cells (Figures 3(a) and 3(b)) while only 6% of cells treated with Protandim prior to H2O2 were apoptotic (P < 0.01 compared to vehicle control) (Figures 3(a) and 3(b)).
Figure 3.
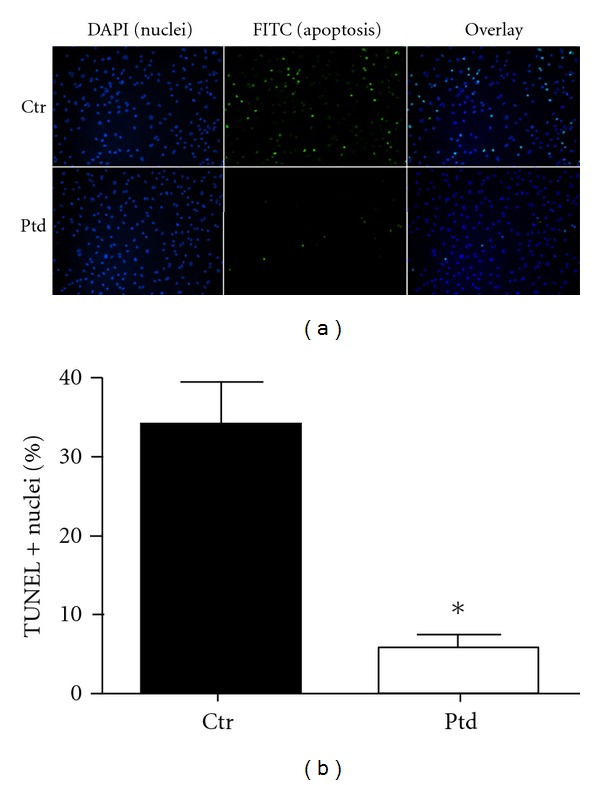
Protandim treatment protects HCAEC against an oxidative stress. HCAEC were treated with Protandim for 12 hrs, followed by H2O2 4 hrs. (a) Treatment of HCAEC with Protandim prior to an oxidative challenge resulted in significantly fewer apoptotic nuclei as determined by TUNEL assay. (b) Approximately 35% of cells underwent apoptosis without Protandim treatment prior to an oxidative challenge while pre-treatment with Protandim resulted in approximately 5% of cells undergoing apoptosis in response to the same oxidative challenge (n = 18 fields) *P < 0.01 compared to control (no Protandim). Data are presented as mean + SE percent of TUNEL positive (+) nuclei. Ptd: Protandim, Ctr: no Protandim.
3.4. Nrf2 Silencing Diminishes Protandim-Induced Increases in HO-1 and Protection from an Oxidative Challenge
Nrf2 was silenced using siRNA prior to Protandim treatment to determine if Protandim induced increases in antioxidant enzyme expression and protection occur through Nrf2 activation. Nrf2 silencing prior to Protandim treatment significantly inhibited (P < 0.01) Protandim-induced HO-1 protein expression compared to both the no RNA condition, and control RNA condition. (Figures 4(a) and 4(b)). There were no differences between no RNA and control RNA conditions with or without Protandim.
Figure 4.
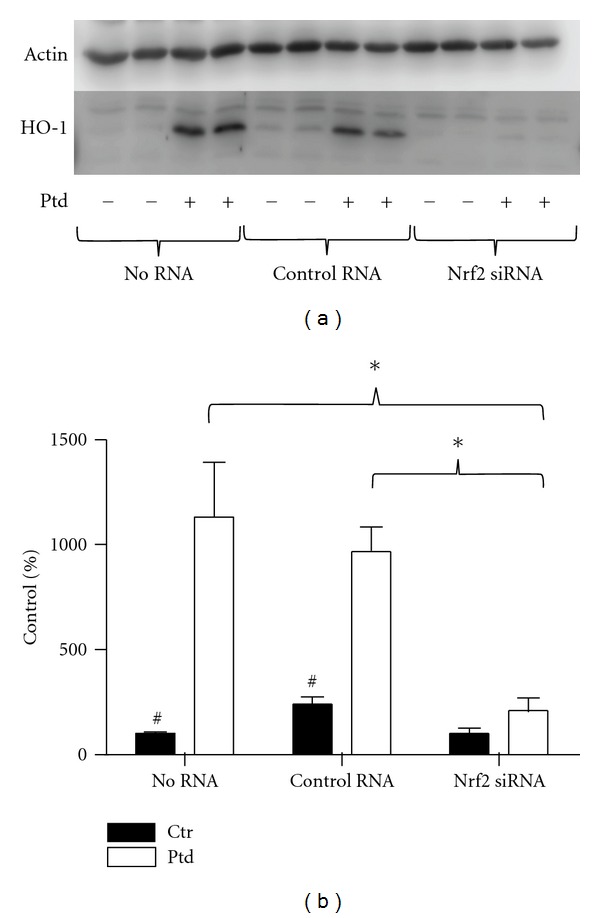
Silencing of Nrf2 abrogated Protandim-induced increases in HO-1 expression. (a) Cells were treated with no RNA, control RNA, or Nrf2 siRNA prior to 12 hrs Protandim, then HO-1 expression determined by Western blotting. (b) HO-1 expression in response to Protandim treatment was elevated over 1,000% of control in the no RNA and control RNA conditions, while increases in HO-1 expression in the Nrf2 siRNA condition were negligible. (n = 6 treatments per condition). Data are presented as mean + SE percent of control (no RNA, no Protandim) condition. *P < 0.01 # P < 0.01 compared to within RNA condition Protandim treatment. Ptd: Protandim, Ctr: No Protandim.
Nrf2 was then silenced prior to Protandim treatment and an oxidative challenge. In cells receiving no RNA and cells that received control RNA, 30–40% of cells underwent apoptosis (Figures 5(a) and 5(b). Nrf2 siRNA treatment prior to H2O2 resulted in apoptosis in 80% of cells (P < 0.0001) compared to no RNA conditions and control RNA (Figures 5(a) and 5(b)). In both no RNA and control RNA conditions, Protandim treatment prior to H2O2 resulted in significantly fewer apoptotic cells (P < 0.0001) (Figure 5(b)). The number of apoptotic cells in the no RNA condition compared to the control RNA condition was not significantly different with or without Protandim (P = 0.413 no Protandim, P = 0.093 with Protandim). Protandim prior to Nrf2 siRNA also significantly protected cells from apoptosis (P = 0.023); however the amount of protection afforded by the Protandim in this condition was significantly less than in no RNA and control RNA conditions (P < 0.01 and P < 0.05, resp.) (Figure 5(c)).
Figure 5.
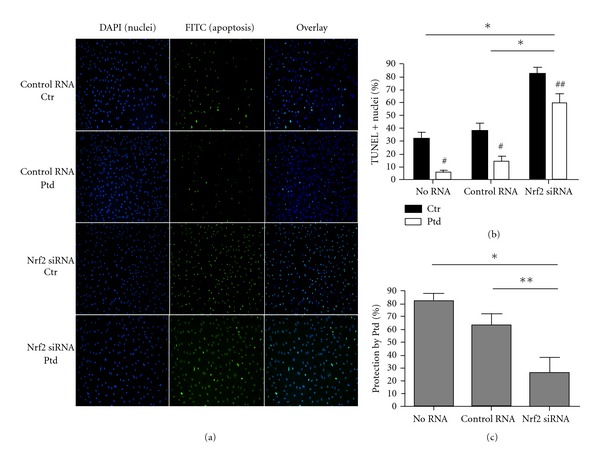
Protandim treatment following Nrf2 silencing protected HCAEC against an oxidative challenge; however, protection following Nrf2 silencing was significantly diminished compared to controls. (a) Silencing of Nrf2 resulted in a significant increase in the number of cells undergoing apoptosis in response to an oxidative challenge regardless of Protandim. (b) In no RNA, control RNA, and Nrf2 RNA conditions, significantly fewer cells underwent apoptosis when treated with Protandim prior to an oxidative stress. The no RNA condition and control RNA were not significantly different from each other with or without Protandim. In Nrf2 siRNA conditions, % TUNEL-positive (+) nuclei were significantly higher than no RNA and control RNA conditions with and without Protandim- (n = 15–19 fields) (c) The percent protection by Protandim was not significantly different between no RNA and control RNA conditions. The protection afforded by Protandim in the Nrf2 siRNA condition was significantly less compared to both no RNA and control RNA conditions. Data are presented as means + SE. *P < 0.01 between indicated groups, **P < 0.05 between indicated groups. # P < 0.01 compared to within RNA condition control (no Protandim), ## P < 0.05 compared to within RNA condition control (no Protandim). Ptd: Protandim, Ctr: No Protandim.
4. Discussion
The novel findings of this study were that Protandim treatment of HCAEC induced Nrf2 nuclear localization and phase II antioxidant enzyme expression, Protandim treatment prior to an oxidative challenge was protective against apoptosis, and this protection was dependent on Nrf2. Our data show that the phytochemical components of Protandim increase Nrf2 in the cytosol and nucleus of HCAEC within an hour of treatment. In cardiac myocytes we have observed increases in Nrf2 within 15 minutes of Protandim treatment (unpublished data). Nrf2 remains elevated through treatment durations up to 12 hours, which was the longest duration examined. These data show for the first time an immediate, substantial, and sustained effect of Protandim on Nrf2 in HCAEC.
4.1. Protandim Induction of Antioxidant Enzymes
The components in Protandim work synergistically to activate Nrf2 and induce antioxidant enzyme expression. HO-1 mRNA was increased to 500 percent of control in response to 40 μg/mL Protandim in MIN6 cells and 1,000 percent of control in response to 20 μg/mL Protandim in SK-N-MC cells [16]. However, when each phytochemical component of Protandim was tested individually at the low concentration found in Protandim extract, the maximum increase in HO-1 mRNA was less than 200% of control [16]. While we observed significant increases in protein expression of multiple antioxidant enzymes (approximately 125% of control for SOD1, GR, and NQO1), the Protandim-induced increase in HO-1 was over 700% of control. Until recently, the antioxidant properties of HO-1 were thought to be through production of the ROS scavenger bilirubin, but data now suggest HO-1 may have other antioxidant qualities and be important in multiple cell types in the vasculature. Kadl et al. found that cell death in macrophages was exacerbated in the absence of Nrf2 or with inhibition of HO-1 activity [21]. It has also been demonstrated in macrophages that HO-1 functions as an antioxidant by decreasing heme availability, which decreases expression of the NADPH oxidase heme containing subunit gp91phox and subsequently decreases superoxide production [28].
Decreasing NOX superoxide production has important implications in both macrophages and endothelial cells. Substantial evidence exists demonstrating a role for NOX and increased superoxide production in obesity and atherosclerosis. Compared to nonobese controls, overweight, and obese subjects demonstrate increased NOX subunit expression and augmented oxidative stress in the vascular endothelium [29]. NOX subunit expression is elevated in lesions of coronary arteries in bypass graft patients, particularly in the vicinity of macrophages, and NOX expression levels correlate with severity of atherosclerosis [10]. Superoxide produced by any mechanism including NOX is rapidly converted to H2O2. Our experiments used H2O2 as an oxidative stress to induce apoptosis in HCAEC. While the concentration of H2O2 used in our experiments was chosen based on consistent induction of apoptosis in 30–50% of untreated cells, H2O2 is a relevant oxidant in vivo [30].
Whether HO-1 also functions as a protective antioxidant in endothelial cells through decreasing heme availability, inhibiting NOX, and decreasing superoxide production, or via an alternative mechanism also is yet to be determined. Greater baseline HO-1 expression in aortic endothelial cells also results in decreased effects of oxidized phospholipids on inflammatory genes [31], suggesting that presence of HO-1 may delay progression of disease-related phenotypic changes when cells are faced with chronic lipid and oxidative stresses. While it was beyond the scope of this study, determining whether HO-1 is essential to the Nrf2-dependent Protandim induced protection against an oxidative challenge is warranted.
SOD1, GR, and NQO1 were also significantly elevated in HCAEC in response to Protandim though the effect was not as large as that seen with HO-1. These are the first data indicating increases in GR and NQO1 in response to Protandim; increases in SOD1 expression and activity have been previously reported [15, 27]. In erythrocytes isolated from human subjects following 120 days of Protandim supplementation, SOD activity was increased by 30% [15]. In an ex vivo preparation of human saphenous veins, Protandim treatment increased SOD activity 3-fold, HO-1 activity 7-fold, and catalase activity 12-fold [27]. Catalase has been shown to be increased by Protandim and to mediate protective effects in erythrocytes and saphenous vein preparations, as well as protect against skin cancer development in an animal model [15, 27, 32]. Collectively, these data suggest that the antioxidant enzymes increase following Protandim treatment in a cell type specific manner.
4.2. Nrf2 Mediated Protection against an Oxidative Challenge by Protandim
Protandim treatment protected HCAEC from H2O2-induced apoptosis, an effect that was significantly diminished but not abrogated with silencing of Nrf2 prior to Protandim treatment. There are multiple potential explanations for this observation. First, diminished but incomplete protection may be due to the rapid turnover rate in Nrf2. Nrf2 is constitutively expressed and rapidly degraded. In unstimulated cells the half-life of Nrf2 is less than 30 minutes [33]. The delivered Nrf2 siRNA may be diminished prior to the completion of Protandim and H2O2 treatments as Nrf2 is rapidly turned over. Second, the amount of siRNA that can be delivered is limited by the ability of the cells to tolerate the transfection procedure. The capacity of the delivered Nrf2 siRNA to silence Nrf2 may be exceeded by the previously noted continuous turnover and subsequent increases in response to Protandim. Finally, antioxidant enzymes can also be activated independently of Nrf2 by other proteins including p53 and sirtuins [34, 35]. However, our data show that significantly more cells undergo apoptosis in response to an oxidative challenge if Nrf2 is silenced regardless of Protandim treatment indicating the importance of Nrf2 in cytoprotection.
We used apoptosis in endothelial cells as an outcome because it has direct translation to vascular disease development and outcomes. Endothelial cell apoptosis contributes to plaque progression and rupture and may be an independent risk factor for thrombosis [36]. Endothelial cell apoptosis can be induced by oxidized lipids as well as by ROS, and the role of endothelial cell apoptosis in atherosclerosis has been extensively reviewed [37–39].
4.3. Translation and Future Studies
Our data suggest positive effects of Protandim in healthy coronary artery endothelial cells supporting future examination of how Protandim may affect cells that have been chronically exposed to oxidative and lipid challenges. Most individuals experience transient and/or chronic lipid and oxidative stress in the vasculature throughout life, and it is the accumulated effects that eventually lead to overt vascular disease. Therefore, it is of interest to determine if Protandim can slow or reverse disease related endothelial cell phenotypic changes using in vivo models of chronic oxidative stress.
Supplementation with Protandim in humans is safe, with no reported adverse side effects [15, 16]. It has been shown that with oral Protandim supplementation in humans, circulating TBARs, a measure of lipid peroxidation, decrease in 5–12 days, an effect that persists with continued supplementation as measured at 30 and 120 days [15]. In addition, erythrocytes isolated from subjects who ingested Protandim for 120 days had greater SOD and catalase activity compared to controls [15]. The dose ingested by human subjects [15] induces similar increases in antioxidant enzymes compared to the 20 μg/mL concentration used in our experiments. Thus the effects observed in our cell culture model directly mirror those seen in vivo indicating that exposure of cells to the components of Protandim in human subjects is likely similar to what we used in vitro. Studies in vivo will need to be performed to determine if these results are translatable to intact coronary arteries.
5. Conclusion
Our current investigation shows for the first time that Protandim treatment in HCAEC induces Nrf2 nuclear localization, phase II antioxidant enzyme expression, and Nrf2-dependent protection from an oxidant stress. Oxidative stress has a well-established role in CAD initiation and progression, and our data support further research on phytochemical activation of Nrf2 and the endogenous antioxidant response as a potential therapeutic approach.
Authors' Contribution
E. L. Donovan, D. j. Reuland, B. F. Miller, and K. L. Hamilton designed research; E. D. conducted research; J. M. McCord provided essential reagents; E. L. Donovan, B. F. Miller, and K. L. Hamilton analyzed data and performed statistical analysis; E. L. Donovan wrote paper. All authors have read and approved the final manuscript.
Acknowledgments
The authors thank Laurie Biela and Dr. Manfred Diehl for their assistance. Funding was provided by CSU Proteomics and Metabolomics Facility Academic Enrichment Program and a Colorado State University CAHS minigrant.
Abbreviations
- HO-1:
Heme oxygenase-1
- Nrf 2:
NF-E2-related factor 2
- ROS:
Reactive oxygen species
- SOD:
Superoxide dismutase
- GR:
Glutathione reductase
- NQO1:
NAD(P)H dehydrogenase [quinone] 1
- Keap-1:
Kelch-like ECH-associated protein 1
- CAD:
Coronary artery disease
- HCAEC:
Human coronary artery endothelial cells.
References
- 1.Walter MF, Jacob RF, Jeffers B, et al. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. Journal of the American College of Cardiology. 2004;44(10):1996–2002. doi: 10.1016/j.jacc.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Robertson AP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. Journal of Biological Chemistry. 2004;279(41):42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 3.Rottkamp CA, Nunomura A, Raina AK, Sayre LM, Perry G, Smith MA. Oxidative stress, antioxidants, and Alzheimer disease. Alzheimer Disease and Associated Disorders. 2000;14(supplement 1):S62–S66. doi: 10.1097/00002093-200000001-00010. [DOI] [PubMed] [Google Scholar]
- 4.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. American Journal of Cardiology. 2003;91(3) doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 5.Carr AC, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(7):1716–1723. doi: 10.1161/01.atv.20.7.1716. [DOI] [PubMed] [Google Scholar]
- 6.Judkins CP, Diep H, Broughton BRS, et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE -/- mice. American Journal of Physiology, Heart and Circulatory Physiology. 2010;298(1):H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Gharavi NM, Honda H, et al. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radical Biology and Medicine. 2009;47(2):145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouhanizadeh M, Hwang J, Clempus RE, et al. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: implication of NADPH oxidase. Free Radical Biology and Medicine. 2005;39(11):1512–1522. doi: 10.1016/j.freeradbiomed.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landmesser U, Merten R, Spiekermann S, Büttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000;101(19):2264–2270. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 10.Sorescu D, Weiss D, Lassègue B, et al. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105(12):1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 11.Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(12):2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 12.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Journal of the American Medical Association. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 13.Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392(6676):p. 559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 14.Clarke R, Armitage J. Antioxidant vitamins and risk of cardiovascular disease. Review of large-scale randomised trials. Cardiovascular Drugs and Therapy. 2002;16(5):411–415. doi: 10.1023/a:1022134418372. [DOI] [PubMed] [Google Scholar]
- 15.Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Free Radical Biology and Medicine. 2006;40(2):341–347. doi: 10.1016/j.freeradbiomed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 16.Velmurugan K, Alam J, McCord JM, Pugazhenthi S. Synergistic induction of heme oxygenase-1 by the components of the antioxidant supplement Protandim. Free Radical Biology and Medicine. 2009;46(3):430–440. doi: 10.1016/j.freeradbiomed.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 17.Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods in Molecular Biology. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- 18.Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry. Biokhimiia. 2006;71(9):962–974. doi: 10.1134/s0006297906090033. [DOI] [PubMed] [Google Scholar]
- 19.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Medica. 2008;74(13):1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 20.He M, Siow RCM, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(4):277–285. doi: 10.1016/j.numecd.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circulation Research. 2010;107(6):737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warabi E, Takabe W, Minami T, et al. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radical Biology and Medicine. 2007;42(2):260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Jyrkkänen HK, Kansanen E, Inkala M, et al. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circulation Research. 2008;103(1):e1–e9. doi: 10.1161/CIRCRESAHA.108.176883. [DOI] [PubMed] [Google Scholar]
- 24.Ungvari Z, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. American Journal of Physiology - Heart and Circulatory Physiology. 2011;300(4):H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H, Tian X, Guo Y, Duan W, Bu H, Li C. Activation of nuclear factor erythroid 2-related factor 2 cytoprotective signaling by curcumin protect primary spinal cord astrocytes against oxidative toxicity. Biological and Pharmaceutical Bulletin. 2011;34:1194–1197. doi: 10.1248/bpb.34.1194. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Hou W, Yao P, et al. Heme oxygenase-1 mediates the protective role of quercetin against ethanol-induced rat hepatocytes oxidative damage. Toxicol in Vitro. 2012;26(1):74–80. doi: 10.1016/j.tiv.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Joddar B, Reen RK, Firstenberg MS, et al. Protandim attenuates intimal hyperplasia in human saphenous veins cultured ex vivo via a catalase-dependent pathway. Free Radical Biology and Medicine. 2011;50(6):700–709. doi: 10.1016/j.freeradbiomed.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Taillé C, El-Benna J, Lanone S, et al. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. Journal of Biological Chemistry. 2004;279(27):28681–28688. doi: 10.1074/jbc.M310661200. [DOI] [PubMed] [Google Scholar]
- 29.Silver AE, Beske SD, Christou DD, et al. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47phox expression and evidence of endothelial oxidative stress. Circulation. 2007;115(5):627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- 30.Brandes RR, Schröder K. Differential vascular functions of Nox family NADPH oxidases. Current Opinion in Lipidology. 2008;19(5):513–518. doi: 10.1097/MOL.0b013e32830c91e3. [DOI] [PubMed] [Google Scholar]
- 31.Romanoski CE, Che N, Yin F, et al. Network for activation of human endothelial cells by oxidized phospholipids: a critical role of heme oxygenase 1. Circulation Research. 2011;109:e27–e41. doi: 10.1161/CIRCRESAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Gu X, Robbins D, et al. Protandim, a fundamentally new antioxidant approach in chemoprevention using mouse two-stage skin carcinogenesis as a model. PloS one. 2009;4(4, article e5284) doi: 10.1371/journal.pone.0005284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maher J, Yamamoto M. The rise of antioxidant signaling-The evolution and hormetic actions of Nrf2. Toxicology and Applied Pharmacology. 2010;244(1):4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Hussain SP, Amstad P, He P, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Research. 2004;64(7):2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- 35.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu F, Sun Y, Chen Y, et al. Endothelial cell apoptosis is reponsible for the formation of coronary thrombotic atherosclerotic plaques. Tohoku Journal of Experimental Medicine. 2009;218(1):25–33. doi: 10.1620/tjem.218.25. [DOI] [PubMed] [Google Scholar]
- 37.Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. Journal of Cellular and Molecular Medicine. 2010;14(1-2):70–78. doi: 10.1111/j.1582-4934.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxidants and Redox Signaling. 2009;11(4):791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoneman VEA, Bennett MR. Role of apoptosis in atherosclerosis and its therapeutic implications. Clinical Science. 2004;107(4):343–354. doi: 10.1042/CS20040086. [DOI] [PubMed] [Google Scholar]


