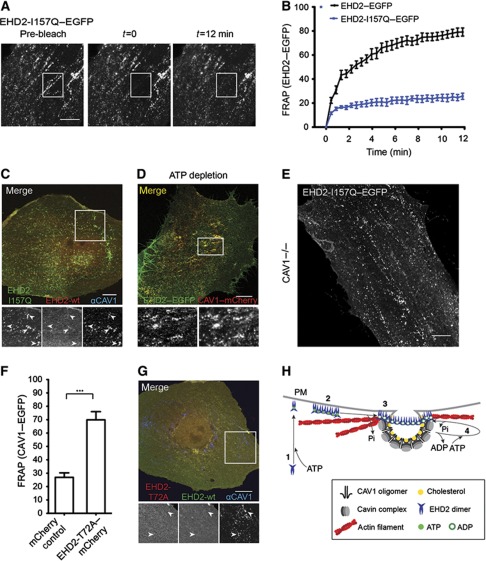
Figure 6.
The ATPase domain regulates EHD2 dynamics and function. (A) Representative confocal images of FRAP experiments with EHD2-I157Q–EGFP expressing cells. No fluorescence recovery at initially bleached spots can be detected during the recorded time. (B) FRAP curves of EHD2–EGFP or EHD2-I157Q–EGFP-transfected CV1 cells (as in Figure 4D). Data points present mean values±s.e.m., n=10 or 8 for EHD2–EGFP or EHD2-I157Q–EGFP. (C) Representative confocal image of a CV1 cell co-expressing EHD2-I157Q–EGFP and EHD2–mCherry for 12 h. Cells were fixed and stained for endogenous CAV1. Arrows in the magnification highlight EHD2-I157Q-positive spots that have weak/no EHD2 wild-type staining and colocalize with CAV1. (D) CV1 cells transfected with EHD2–EGFP and CAV1–mCherry were incubated for 40 min in ATP-depletion media and analysed by confocal microscopy. Magnification highlights colocalization of EHD2 and CAV1. (E) Confocal image of MEF CAV1−/− cell transfected with EHD2-I157Q–EGFP for 6 h. (F) Relative CAV1 fluorescence 12 min after bleaching peripheral regions in HeLa cells, stably expressing CAV1–EGFP and transfected with mCherry control (n=11) or EHD2-T72A–mCherry (n=11) plasmids. Significance of mean differences between the conditions was calculated with a two-tailed unpaired t-test. ***P-value<0.0001. (G) Representative confocal image of a CV1 cell co-expressing EHD2–EGFP and EHD2-T72A–mCherry. Cells were fixed and stained for endogenous CAV1. Arrows in the magnification highlight that CAV1-positive spots are devoid of wild-type EHD2 and EHD2-T72A. All scale bars 10 μm. (H) A model on the role of oligomer formation and the ATP cycle in EHD2 association with caveolae and dynamic exchange. (1) Newly synthesized EHD2 dimers bind ATP and are subsequently targeted to the plasma membrane via ionic interactions with negatively charged lipids. (2) Upon lipid binding, EHD2 dimers assemble into 60–75S oligomers via interactions of EH domain and KPF/NPF motifs in adjacent EHD2 dimers. (3) Association of EHD2 complexes with caveolae requires ATP hydrolysis, which may be stimulated at caveolae. (4) EHD2 proteins undergo dynamic exchange at caveolae, which requires a new cycle of ATP binding and hydrolysis. EHD2 mediates a link to actin fibres that run in close proximity to caveolae.