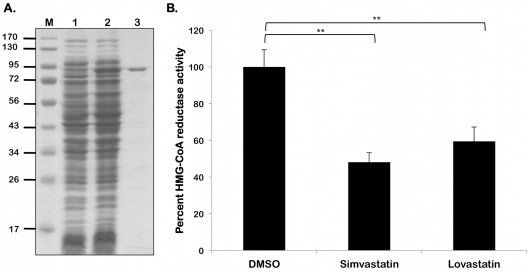
Figure 3. Statins inhibit the enzyme activity of B. burgdorferi HMGR.
(A) Lysates purified HMGR as indicated above the respective lanes were resolved by SDS-12.5%PAGE. Gels were stained with Coomassie blue. Recombinant HMGR of B. burgdorferi purified by FPLC. Lane 1, non-induced E. coli containing pMAL-p2X/hmgr. Lane 2, E. coli containing pMAL-p2X/hmgr induced with 1 mM IPTG. Lane 3, Purified MBP-HMGR fusion protein. (B) Recombinant B. burgdorferi HMGR activity was measures as described under Materials and Methods. Statins were added to the enzyme reaction at a concentration of 250 µM. The velocity of the control reaction was calculated as 1.41±0.10 µmol NADPH oxidized minute−1 (mg protein)−1, set as 100% maximal activity in the presence of the statin diluent, DMSO, alone. Simvastatin lowered HMGR activity to 48.2% of maximal (velocity of 0.87±0.08 µmol NADPH oxidized minute−1 (mg protein)−1), while lovastatin reduced HMGR activity to 61.4% of maximal activity (velocity of 0.68±0.07 µmol NADPH oxidized minute−1 (mg protein)−1). Data shown are the average of three independent assays. Asterisks indicate samples whose values are statistically significantly different between control and treated conditions (**, P<0.01).