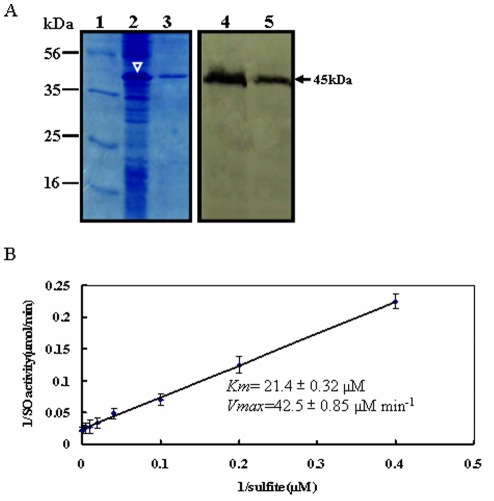
Figure 1. Expression, purification and kinetic analysis of recombinant maize sulfite oxidase.
(A) Purification of histidine-tagged maize SO. The histidine-tagged ZmSO (with a predicted molecular mass of 45 kDa) was overexpressed in bacterial cells induced by isopropyl-β-D-thiogalactopyranosid (lane 2, indicated by white arrowhead). The overexpressed protein was purified using metal chelate affinity chromatography (lane 3). The overexpressed and purified SO proteins were confirmed by Western blot with an anti-histidine monoclonal antibody (lane 4 and lane 5; signals marked by an arrowhead). The molecular mass markers (kDa, lane 1) are shown on the left. (B) Steady-state kinetics of recombinant ZmSO with sulfite. Double-reciprocal presentation (Lineweaver-Burk plot) of enzyme rate was conducted using varying concentrations of sulfite (2.5, 5, 10, 25, 50, 100, 200, and 400 µM) and constant 400 µM ferricyanide. The reaction was initiated with 1.0 µg of purified recombinant ZmSO.