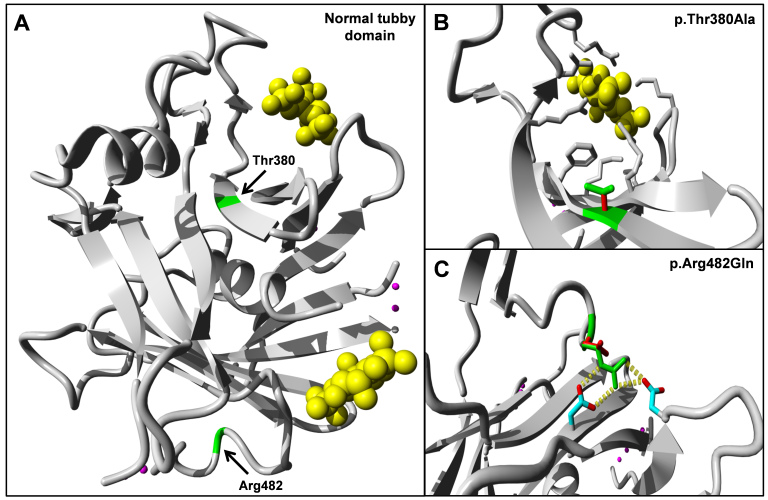
Figure 5.
Three-dimensional domain architecture of the tubby domain of TULP1 wild-type and mutant proteins. A: Preferred predicted secondary structure of normal tubby-like protein 1 (TULP1) with Thr380 and Arg482 indicated in green. In yellow, the inositol triphosphate molecules that are predicted to bind the tubby domain of TULP1. B: Predicted structure of part of the p.Thr380Ala mutant protein in affected individuals of family A. The smaller size of the alanine residue may lead to rearrangements of surrounding residues and thereby affect putative inositol triphosphate binding. C: Part of the predicted structure of the p.Arg482Gln mutant protein found in affected individuals of family B. The p.Arg482Gln variant changes a positively charged amino acid (arginine) to a neutral residue (glutamine), which leads to loss of interactions with two negatively charged residues in its vicinity. Wild-type interactions are indicated with yellow blocks.