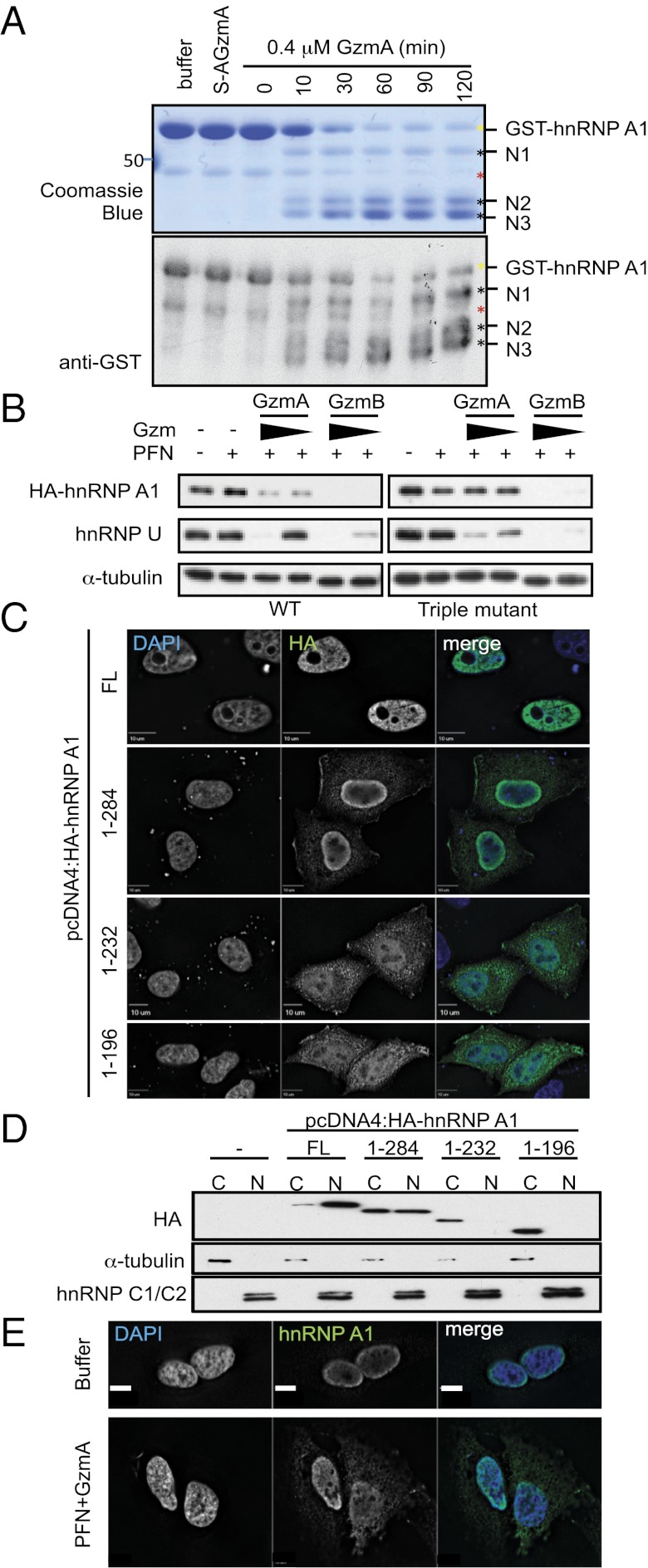
Fig. 5.
GzmA cleaves hnRNP A1 after R196, R232, and R284, resulting in cytoplasmic mislocalization. (A) Recombinant purified GST-hnRNP A1 was treated with 0.4 μM GzmA or S-AGzmA and examined by Coomassie blue (Upper) or GST immunoblot (Lower). The GST tag was at the N terminus. Three N-terminal cleavage products (black asterisks) appear within 10 min. Yellow asterisk indicates full-length GST-hnRNP A1; red asterisk indicates a contaminating band. Mass spectrometry of excised bands indicated cleavage after R196, R232, and R284. (B) HeLa cells, expressing WT (Left) or GzmA-uncleavable mutant (Right) HA-hnRNP A1, were treated with PFN plus GzmA or GzmB (0.5 and 0.16 μM, respectively) for 1 h. HA-hnRNP A1 cleavage was assessed by immunoblot probed for HA. α−Tubulin was the loading control, and endogenous hnRNP U served as a cleavage control. WT HA-hnRNP A1 and endogenous hnRNP U were cleaved by both Gzms; mutant HA-hnRNP A1 was resistant to cleavage by GzmA but not by GzmB. (C) HeLa cells expressing full-length (FL) HA-hnRNP A1 or indicated truncations were stained with anti-HA (green) and DAPI (blue). (D) HeLa cells expressing GzmA-generated HA-hnRNP A1 truncations were separated into cytoplasmic (C) and nuclear (N) fractions, and HA-hnRNP A1 localization was assessed by anti-HA immunoblot. Tubulin and hnRNP C1/C2 are fractionation controls for the cytoplasm and nucleus, respectively. (E) Immunofluorescence localization of endogenous hnRNP A1. Untransfected HeLa cells were treated with buffer or PFN plus 1 μM GzmA for 1 h before staining for hnRNP A1 (green) and DAPI (blue). Blots and images are representative of at least three independent experiments.