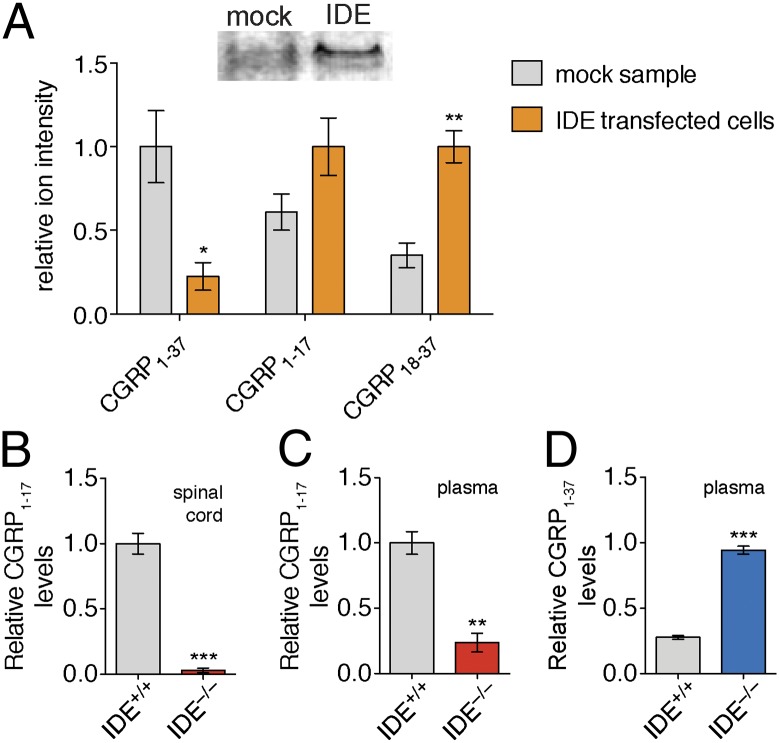
Fig. 4.
IDE cleaves CGRP1–37 in complex proteomes. (A) TT cell line naturally produces and secretes CGRP. By Western blot (Inset, Top) TT cells have very little or no IDE. To study the impact of IDE on CGRP levels in TT cells, we transfected these cells with an empty vector (mock) or a vector containing the IDE gene (IDE transfected cells). CGRP1–37 levels were significantly lower in media from IDE transfected cells, whereas levels of the CGRP fragment, CGRP18–37, were elevated in media expressing IDE, indicating increased CGRP1–37 proteolysis. Incubation of CGRP1–37 with (B) spinal cord lysate and (C) plasma revealed higher CGRP1–17 in IDE+/+ samples, demonstrating that IDE is responsible for the majority of the CGRP-degrading activity in these lysates and plasma. (D) Incubation of CGRP1–37 with plasma revealed that CGRP1–37 is more stable in IDE−/− plasma, demonstrating that IDE processing can regulate CGRP1–37 levels in vitro. Relative quantification was accomplished by using the ion intensity of the different peptides in the LC–MS chromatogram. These ion intensities were then normalized to the largest peak to enable comparison of different peptides on the basis of relative changes in their abundance. (*P value < 0.05, **P value < 0.01, ***P value < 0.001; P values were derived from the two-tailed Student t test, n = 3. Error bars show SEM.)