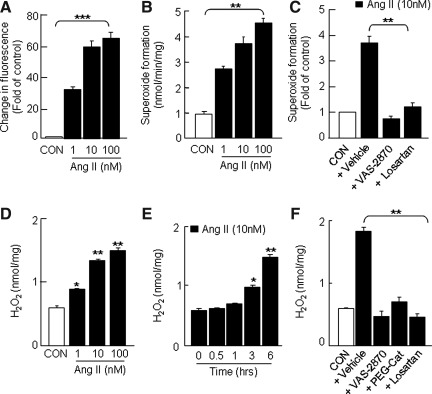
FIG. 2.
Induction of ROS generation in H295R cells by Ang II. Cells, grown in an eight-well glass slide chamber, were loaded with DCFDA (5 μM for 30 min) and then treated with either Ang II (1, 10, and 100 nM) or vehicle (CON) for various periods. Ang II-induced changes in DCFDA fluorescence was monitored by fluorescence microscopy. Concentration-dependent Ang II-induced changes in DCFDA fluorescence (A) and superoxide formation (B) are shown. Effects of VAS-2870 (20 μM; a Nox-specific inhibitor) and losartan (10 μM, an AT1R-specific antagonist) on Ang II (10 nM for 6 h)-dependent superoxide formation (C), concentration- (D), and time (E)-dependent Ang II (10 nM)-induced changes in H2O2 levels. Effects of VAS-2870 (20 μM), PEG-catalase (PEG-Cat, 350 U/ml), and losartan (10 μM) on Ang II (10 nM for 6 h)-dependent H2O2 formation (F) are shown. The incubation time for DCFDA fluorescence and superoxide/H2O2 measurements was 1 and 6 h, respectively. The DCFDA fluorescence intensity was calculated from four different fields, and the data are expressed as fold increase of control (CON=1). Superoxide and H2O2 generation was measured spectrophotometrically by monitoring SOD (2000 U/ml)-inhibitable, NADPH-dependent oxidation of cytochrome C (80 μM) and catalase-inhibitable oxidation of Amplex Red (100 μM) to resorufin, respectively, as described in Materials and Methods section. Results derived from six independent experiments are presented as mean±SEM. *p<0.05, **p<0.01, and ***p<0.001. AT1R, angiotensin II type I receptor; DCFDA, 6-Carboxy- 2′,7′-dichlorodihydrofluorescein diacetate; Nox, NADPH oxidase; ROS, reactive oxygen species; SOD, superoxide dismutase.