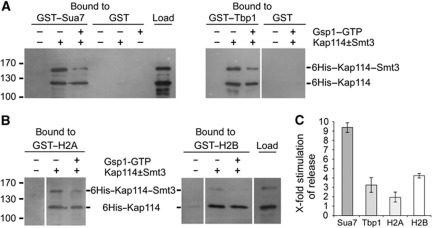
Figure 7.
Sumoylation of Kap114 stimulates the Gsp1–GTP-mediated dissociation of Sua7, Tbp1, and the H2 histones from Kap114. (A, B) Purified 6His–Kap114 was sumoylated in vitro as described in Figure 1A. Subsequently, the mixture (25% of the load is shown) was incubated at 4°C for 1 h with GST–Sua7, GST–Tbp1, and GST (A) or GST–H2A and GST–H2B (B) immobilized on glutathione sepharose. After three washing steps, the reactions were further incubated with buffer alone or with excess amounts of 6His–Gsp1 Q71L–GTP at 4°C for 1 h. After washing, bound proteins were eluted with SDS sample buffer, separated by SDS–PAGE, and detected by western blotting using anti-Kap114 antibodies. Molecular weight markers are shown in kDa. The lanes in each gel block originate from the same gel. (C) The stimulation of Gsp1–GTP-mediated cargo release by sumoylation of Kap114 was quantified. Data are given as x-fold stimulation of release (average values±s.d., n=3) of Sua7, Tbp1, H2A, and H2B from sumoylated versus unmodified Kap114 in the presence of Gsp1–GTP. The ratio of bound unmodified Kap114 after incubation with buffer alone and unmodified Kap114 after incubation with Gsp1–GTP was divided by the ratio of bound sumoylated Kap114 after incubation with buffer alone and sumoylated Kap114 after incubation with Gsp1–GTP. This value defined the factor of x-fold stimulation of cargo release plotted on the y axis. Figure source data can be found with the Supplementary data.