Table 3.
Effects of Catalyst and Additive on Cross-coupling of Aryl Bromides with n-C10H21ZnI.a
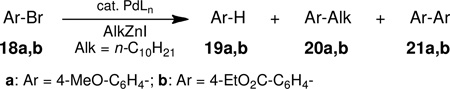 | |||
|---|---|---|---|
| entry | Ar-Br | catalyst | 19 / 20 / 21b |
| 1 | PdCl2(PPh3)2 | < 5% conv | |
| 2 | 18a | PdCl2(dppf) | 7/91/2 |
| 3 | PdCl2(PPh3)2 + TMEDAc | <1/99/0 (95%)d | |
| 4 | PdCl2(PPh3)2 | 4/26/70 | |
| 5 | 18b | PdCl2(dppf) | 5/91/4 |
| 6 | PdCl2(PPh3)2 + TMEDAc | <1/98/1 (93%)d | |
Conditions: alkylzinc iodide (1.1 mmol, 1.0 M in THF), aryl bromide (1 mmol), Pd catalyst (2 mol %). Reactions were run at 0.33 M at 40 °C, 12 h for entries 1–3, and at rt, 20 h for entries 4–6.
by GC on crude material.
1.1 equiv.
Isolated yield.
