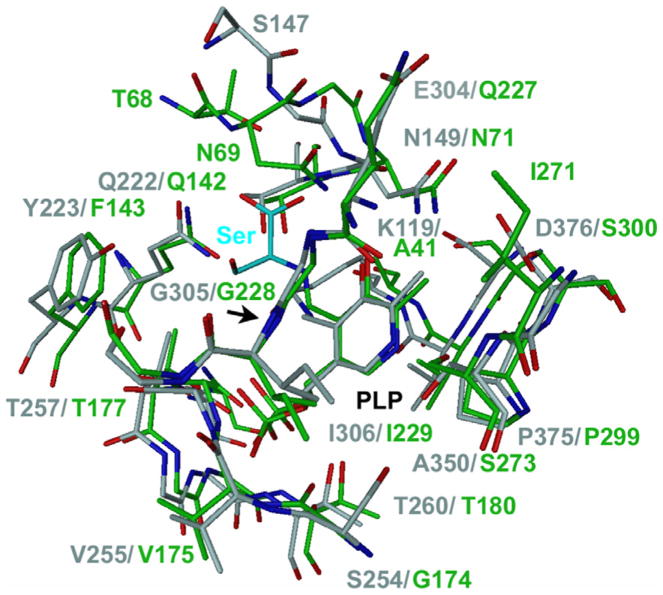
Fig. 5.
Superposition of the active site structures of CBS and the CS. CBS is colored gray and CS green. The active site of CBS is in the open, ligand-free conformation and CS in the closed conformation with a ligand bound. The image was adapted from Fig 3a in [22]. Here, the original substrate analogue methionine in CS has been replaced by a serine in a model-building program. The figure illustrates a putative serine/cysteine-binding site for CBS. Serine and cysteine are similar in size and adopt similar conformations. The methionine in the structure of CS is covalently linked to the PLP as external aldimine. Also the substrate serine or cysteine forms an external aldimine during catalysis. It is therefore likely that they bind at a same location like methionine, considering the high conservation of the active site residues and structure between CBS and CS. For discussion of the active site please see the text.