Table 1.
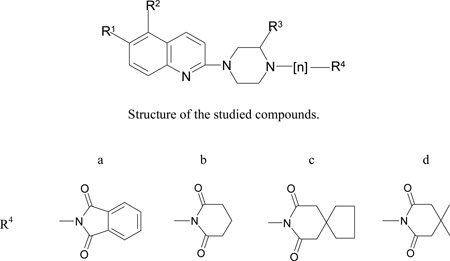
The molecular structure and affinities to SERT (IC50, nM) and 5-HT1A receptor affinities (Ki, nM) of the studied compounds. In the brackets the inhibition constant of [3H]5-HT at human tissue is included.
| Compound | R1 | R2 | R3 | n | R4 | SERT IC50 ± SEM, nM |
5-HT1A Ki ± SEM, nM |
|---|---|---|---|---|---|---|---|
| 1 | NO2 | H | H | - | H | ND (Ki=0.17nM) | ND |
| 2 | H | H | H | - | H | ND (Ki=30 nM) | 230 |
| 3 | NO2 | CH3 | H | 4 | b | 267.8 ± 26.3 | 711.0 ± 21.0 |
| 4 | NO2 | H | H | 4 | b | 921.1 ± 8.2 | 10600 ± 3500 |
| 5 | NO2 | CH3 | H | 4 | c | 1350 ± 211 | 776.5 ± 117.5 |
| 6 | NO2 | H | H | 4 | c | 25900 ± 1100 | 1100 ± 400 |
| 7 | NO2 | CH3 | H | 4 | d | 806.8 ± 85.2 | 221.0 ± 34.6 |
| 8 | NO2 | H | H | 4 | d | 616.3 ± 1.1 | 2100 ± 400 |
| 9 | NO2 | H | H | 4 | a | 4130 ± 900 | 59000 ± 23100 |
| 10 | H | H | Cl | 4 | d | 2800 ± 200 | 1400 ± 100 |
| 11 | C(O)NHCH3 | H | H | 4 | c | 18800 ± 700 | 3200 ± 800 |
| 12 | H | CH3 | H | 4 | a | 33.4 ± 17.0 (8)* | 13[17] |
| 13 | H | H | H | 4 | a | 95.5 ± 37.2 | 39[17] |
| 14 | H | CH3 | H | 3 | a | (80)* | 275[19] |
| 15 | H | CH3 | H | 4 | b | (50)* | 37 ± 6[18] |
| 16 | H | CH3 | H | 4 | c | 75.0 ± 16.3 | 24 ± 3[18] |
| 17 | H | H | H | 4 | c | 111.6 ±27.2 | 39 ± 7[16] |
| 18 | H | CH3 | H | 4 | d | 170.7 ±68.5 | 15 ± 3[18] |
| 19 | H | H | H | 4 | d | 146.4 ±19.4 | 11 ± 2[16] |
data obtained from Servier laboratories