Abstract
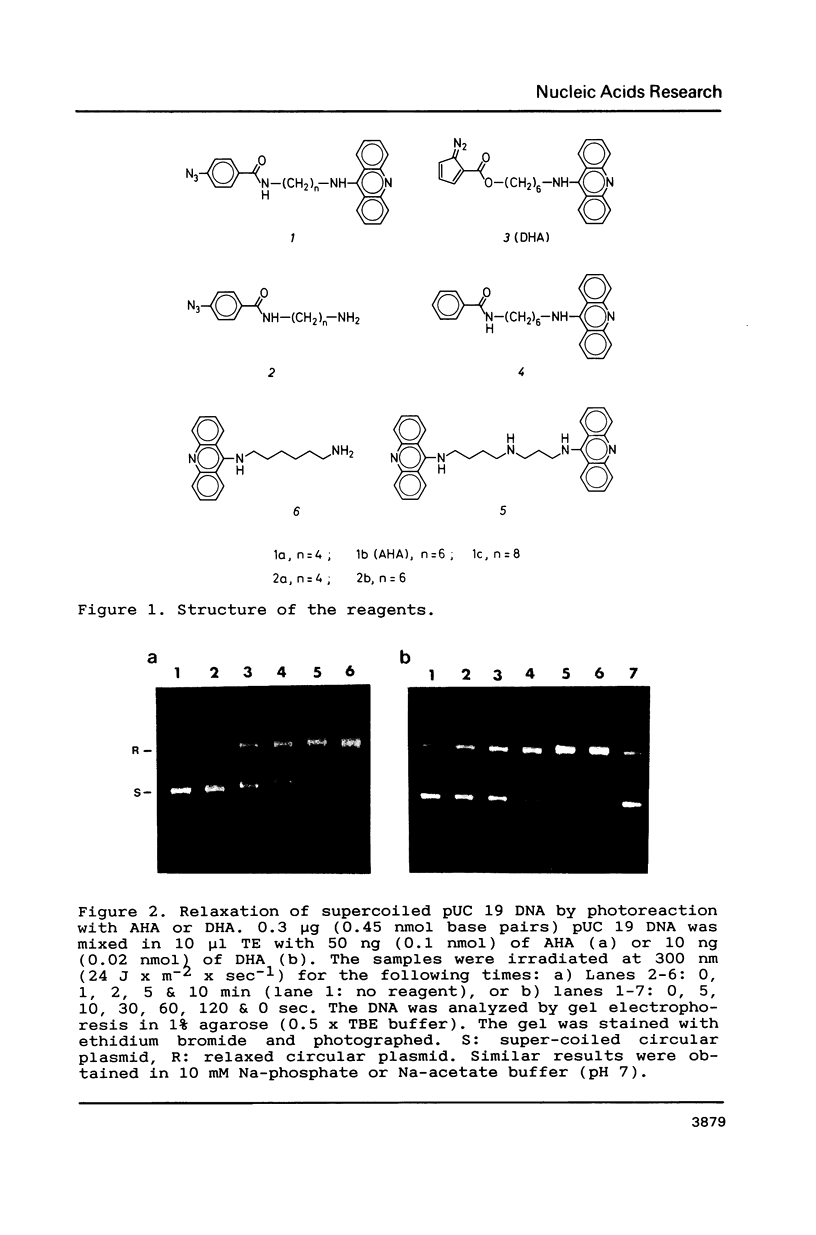
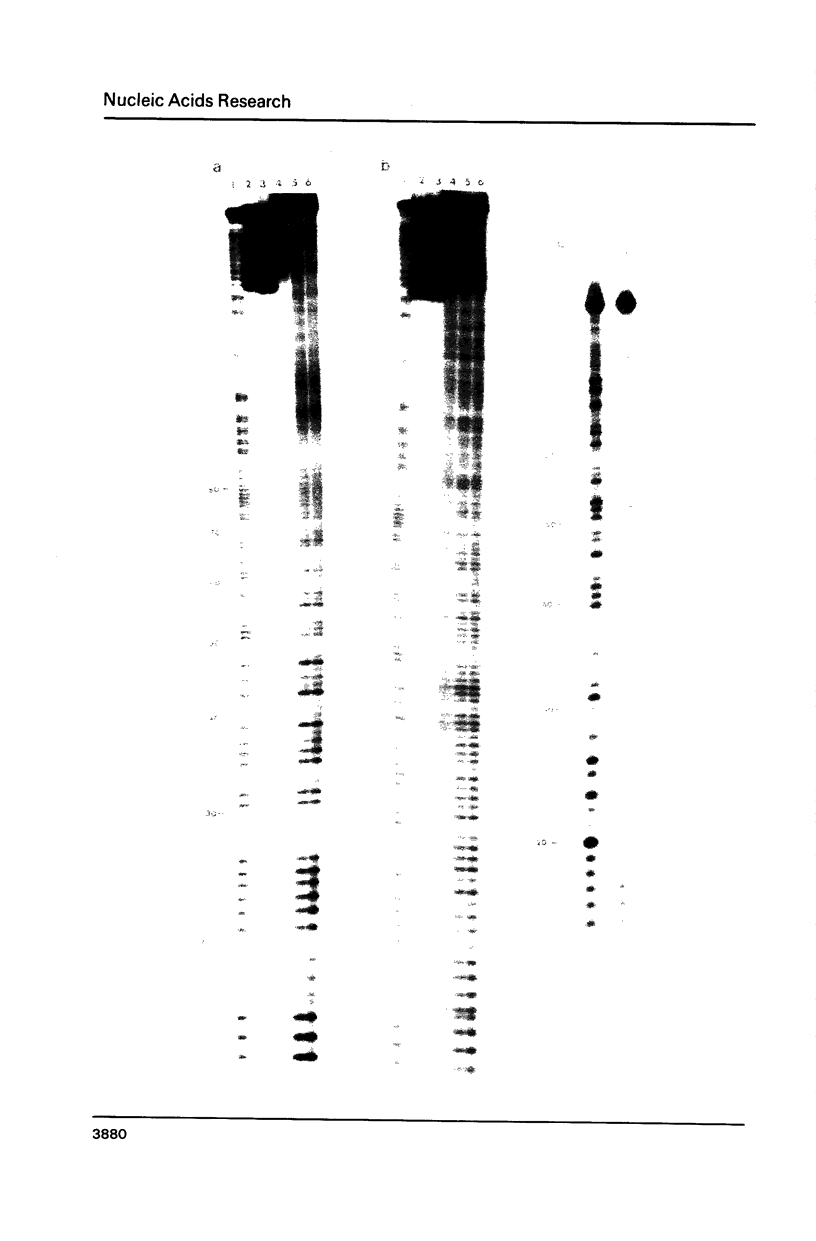
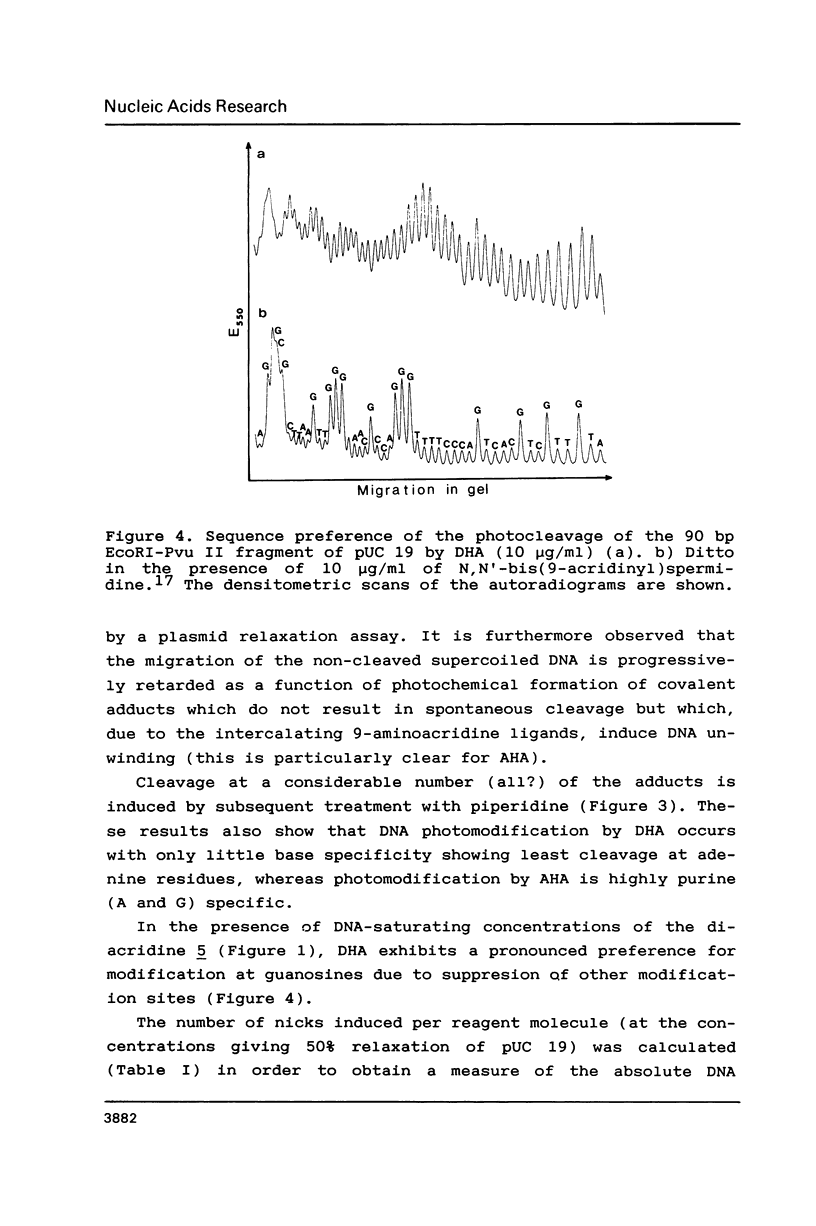
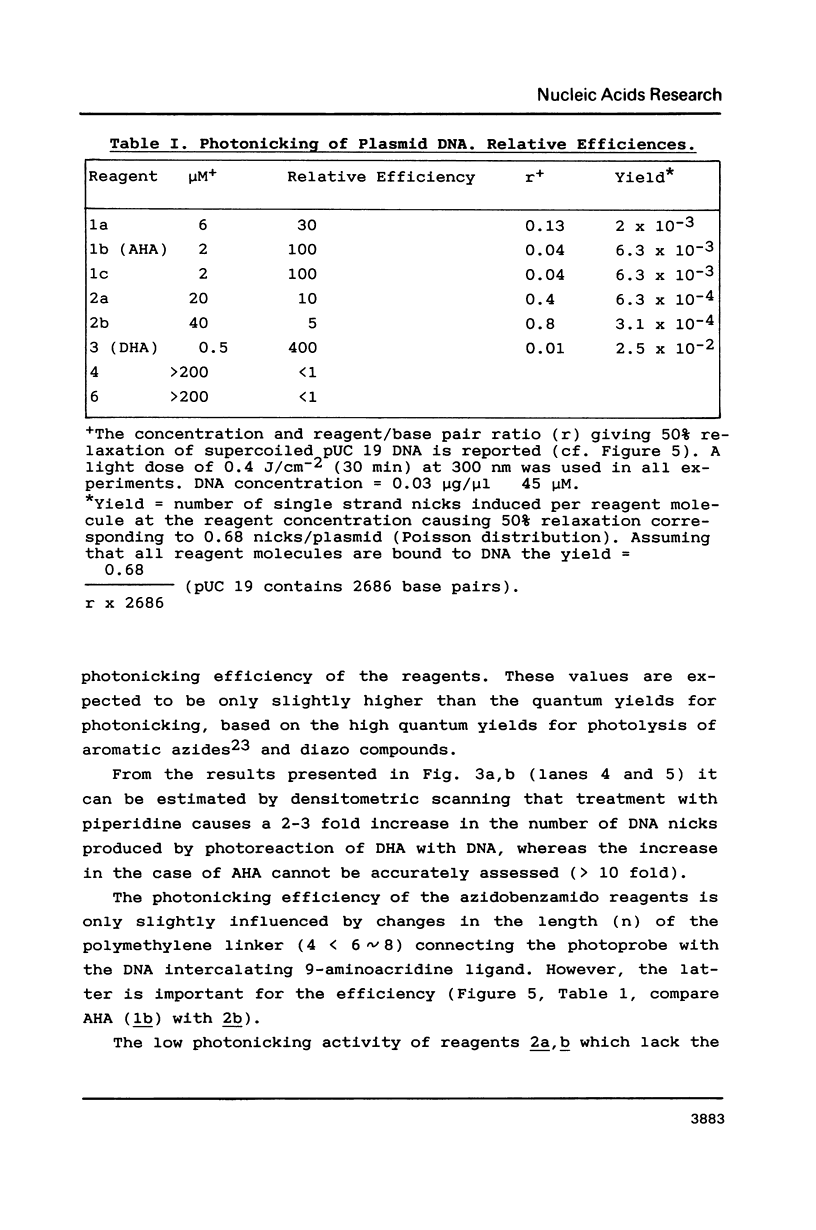
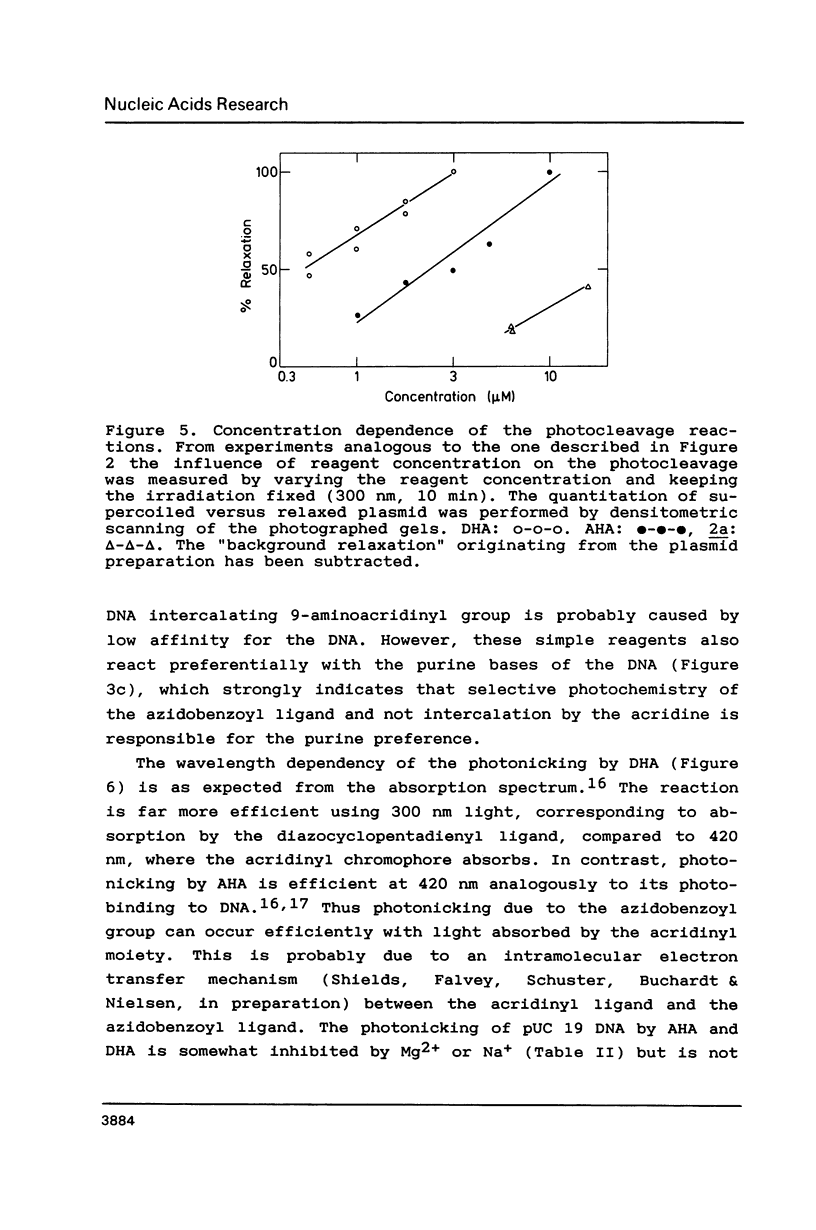
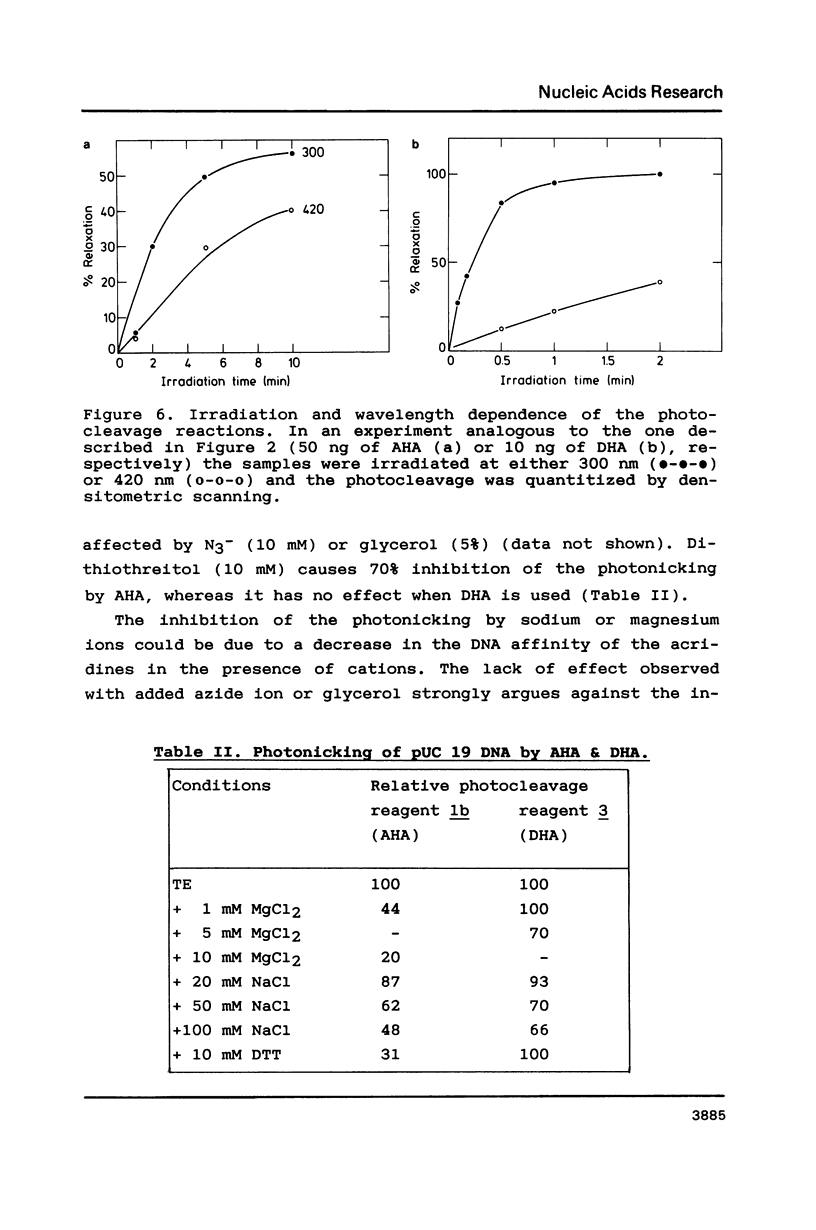
The photoreactions of 9-[6-(4-azidobenzamido)hexylamino]acridine (AHA) and 9-[6-(2-diazocyclopentadienylcarbonyloxy)hexylamino]acridine (DHA) with double stranded DNA result in formation of single strand nicks and alkali labile sites (adducts) with an efficiency of 6 x 10(-3) nicks per AHA and 3 x 10(-2) nicks per DHA molecule. The alkali dependent DNA cleavage by AHA shows a pronounced A+G preference whereas that by DHA is practically sequence independent. In the presence of diacridines, however, DHA exhibits a preference for cleavage at guanosines. These DNA photocleaving reagents could be useful for DNA photofootprinting and photosequencing.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- FREIFELDER D., DAVISON P. F., GEIDUSCHEK E. P. Damage by visible light to the acridine orange--DNA complex. Biophys J. 1961 May;1:389–400. doi: 10.1016/s0006-3495(61)86897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Brown D. M. Base-specific reactions useful for DNA sequencing: methylene blue--sensitized photooxidation of guanine and osmium tetraoxide modification of thymine. Nucleic Acids Res. 1978 Feb;5(2):615–622. doi: 10.1093/nar/5.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Koch T., Buchardt O., Nielsen P. E., Wirth M., Nordén B. Acridine-psoralen amines and their interaction with deoxyribonucleic acid. Biochemistry. 1983 Oct 11;22(21):4878–4886. doi: 10.1021/bi00290a003. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Praseuth D., Habhoub N., Decout J. L., Thuong N. T., Lhomme J., Hélène C. Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[alpha]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987 Oct 12;15(19):7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E. Photoaffinity labeling of chromatin. Synthesis and properties of arylazido derivatives of 9-aminoacridine: potential photolabels for chromatin studies. Eur J Biochem. 1982 Feb;122(2):283–289. doi: 10.1111/j.1432-1033.1982.tb05878.x. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E., Zhen W. P., Henriksen U., Buchardt O. Sequence-influenced interactions of oligoacridines with DNA detected by retarded gel electrophoretic migrations. Biochemistry. 1988 Jan 12;27(1):67–73. doi: 10.1021/bi00401a012. [DOI] [PubMed] [Google Scholar]
- OhUigin C., McConnell D. J., Kelly J. M., van der Putten W. J. Methylene blue photosensitised strand cleavage of DNA: effects of dye binding and oxygen. Nucleic Acids Res. 1987 Sep 25;15(18):7411–7427. doi: 10.1093/nar/15.18.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praseuth D., Chassignol M., Takasugi M., Le Doan T., Thuong N. T., Hélène C. Double helices with parallel strands are formed by nuclease-resistant oligo-[alpha]-deoxynucleotides and oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent with complementary oligo-[beta]-deoxynucleotides. J Mol Biol. 1987 Aug 20;196(4):939–942. doi: 10.1016/0022-2836(87)90416-5. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Gaudemer A., Verlhac J. B., Kraljic I., Sissoëff I., Guillé E. Photocleavage of DNA in the presence of synthetic water-soluble porphyrins. Photochem Photobiol. 1986 Dec;44(6):717–724. doi: 10.1111/j.1751-1097.1986.tb05529.x. [DOI] [PubMed] [Google Scholar]
- Saito I., Sugiyama H., Matsuura T., Ueda K., Komano T. A new procedure for determining thymine residues in DNA sequencing. Photoinduced cleavage of DNA fragments in the presence of spermine. Nucleic Acids Res. 1984 Mar 26;12(6):2879–2885. doi: 10.1093/nar/12.6.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz P. G., Dervan P. B. Sequence-specific double-strand cleavage of DNA by penta-N-methylpyrrolecarboxamide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1983 Nov;80(22):6834–6837. doi: 10.1073/pnas.80.22.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka J. P., Horvath S. J., Bruist M. F., Simon M. I., Dervan P. B. Synthesis of a sequence-specific DNA-cleaving peptide. Science. 1987 Nov 20;238(4830):1129–1132. doi: 10.1126/science.3120311. [DOI] [PubMed] [Google Scholar]
- Spassky A., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper ion. Conformational analysis and footprinting of the lac operon. Biochemistry. 1985 Dec 31;24(27):8050–8056. doi: 10.1021/bi00348a032. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Dervan P. B. Methidiumpropyl-EDTA.Fe(II) and DNase I footprinting report different small molecule binding site sizes on DNA. Nucleic Acids Res. 1983 Aug 25;11(16):5555–5567. doi: 10.1093/nar/11.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]