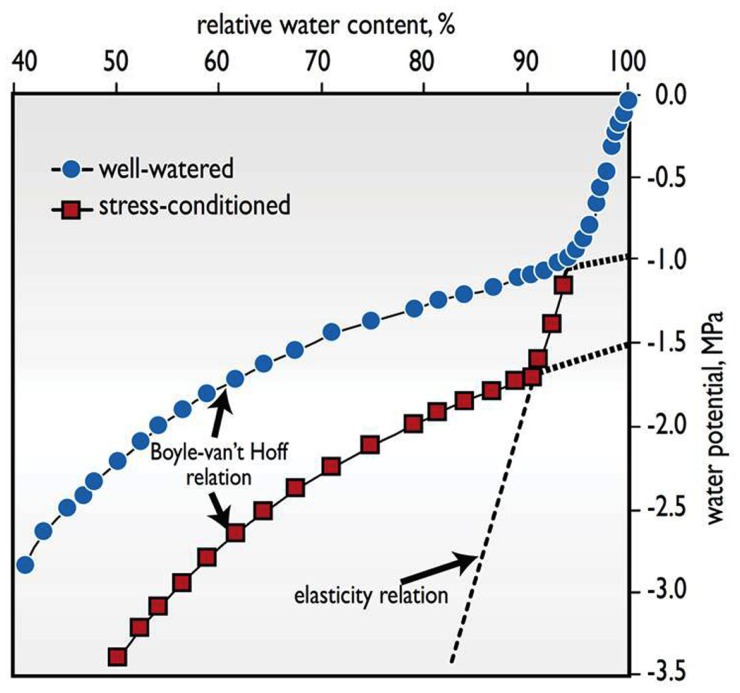
Figure 1.
The advantages of accumulating osmotically active solutes is illustrated with the response of total water potential (Ψw) to a lowering of relative water content (RWC) in wheat leaves, comparing a plant that was well-watered with another that was stress-conditioned by several cycles of water deficit and rewatering over a number of weeks, causing it to accumulate additional osmotically active solutes (Adapted from Melkonian et al., 1982). Two phases are identified. In tissue equilibrated with free water (Ψw ≈ 0 MPa), a large positive turgor pressure balances the osmotic component of water potential. As water is lost, turgor decreases steeply, reflecting the rather stiff cell walls in mature organs, in accordance with the elasticity relation. At RWCs below the point of zero turgor (the inflection point), the relationship is dictated by the less steep Boyle-van’t Hoff relation between osmotic solute activity and water potential, Ψw ≈ −RTC, where R is the ideal gas constant, T is the absolute temperature, and C is the concentration in moles of osmotically active solutes per kg of water. Tissues that accumulate more solutes via the process of osmotic adjustment, such as the stress-conditioned leaf shown here, shift the Boyle-van’t Hoff curve to lower water potentials. As a consequence, the tissue can maintain positive turgor over a wider Ψw range and incur less shrinkage at still lower levels of Ψw below the turgor loss point. Such shrinkage and low Ψw can damage the integrity of cell membranes and other cell constituents, and lead to cell death as in stress-induced leaf necrosis.