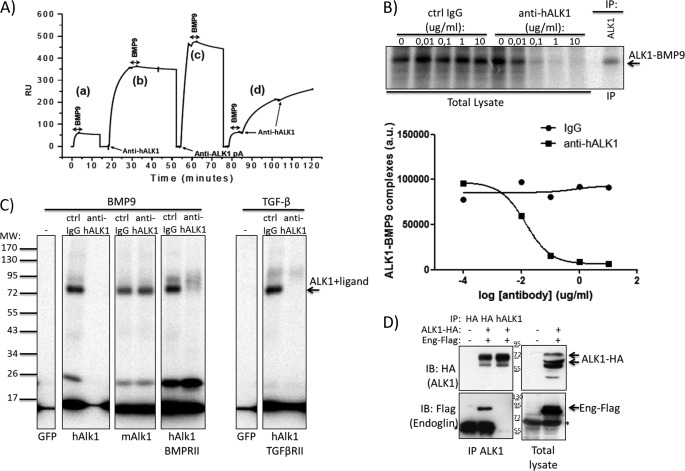
FIGURE 3.
Anti-hALK1 antibody competes with BMP9 for ALK1 binding and prevents heteromeric ALK1-endoglin complex formation. A, human recombinant extracellular domain of ALK1 was immobilized on a Biacore chip to study ALK1 binding by measuring surface plasmon resonance. BMP9 bound immobilized recombinant ALK1 with high affinity (a). Anti-hALK1 antibody binding to recombinant hALK1 blocked BMP9 binding (b). BMP9 binding was partially reduced in the presence of an anti-ALK1 polyclonal antibody (pA) (c). When recombinant ALK1 surface was saturated with BMP9, anti-hALK1 antibody binding was observed (d) with a significantly reduced association rate. RU, relative units. B, 125I-labeled BMP9 was added to HUVECs that were incubated with increasing concentrations of control (Ctrl) IgG or anti-hALK1 antibody (0.01–10 μg/ml). After a 2-h incubation on ice, BMP9 was chemically cross-linked to cell surface proteins. After gel electrophoresis, BMP9-bound complexes were identified by autoradiography. To identify ALK1-BMP9 complexes, ALK1 was immunoprecipitated (IP) from cell lysates. Anti-hALK1 antibody blocked the formation of BMP9-ALK1 complexes at concentrations from 0.1 μg/ml. Quantification of the ALK1-BMP9 band using ImageQuant software (Amersham Biosciences) is shown below. a.u., arbitrary units. C, COS-1 cells were transfected with the indicated plasmids and subsequently incubated with 125I-labeled BMP9 or TGF-β in the presence of a control IgG or anti-hALK1 (10 μg/ml). After a 2-h incubation on ice, BMP9 and TGF-β were chemically cross-linked to cell surface proteins. After gel electrophoresis, ligand-bound receptor complexes were identified by autoradiography. GFP-transfected cells did not bind BMP9 or TGF-β. Human ALK1, mouse ALK1, and the combination of human ALK1 and BMP type II receptor all bound BMP9. The interaction with human ALK1, but not mouse (m) ALK1, was inhibited by the addition of anti-hALK1. TGF-β binding to ALK1 was similarly blocked by addition of anti-hALK1. All panels come from the same gel and were exposed equally long. D, HEK293T cells were either mock- (first lane) or co-transfected with human ALK1-HA antibody and human endoglin-FLAG (second and third lanes). From lysates, ALK1 was successfully precipitated (see upper left panel) either by HA antibody (second lane) or by the anti-hALK1 antibody (third lane). Subsequently, blots were reprobed for co-immunoprecipitated endoglin-FLAG (lower left panel). Strikingly, although anti-HA and anti-hALK1 antibody both immunoprecipitated (IP) equal amounts of ALK1, anti-hALK1 antibody failed to co-immunoprecipitate endoglin (Eng). The right panels show control lysates of mock- (first lane) and co-transfected cells (second lane). Note that mature ALK1 (highest molecular mass) was favorably immunoprecipitated by both the HA and anti-hALK1 antibodies. Numbers indicate the molecular mass marker bands (in kDa), and * denotes nonspecific bands. IB, immunoblot.