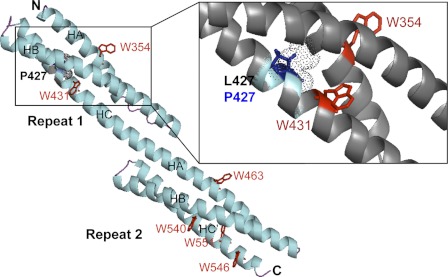
FIGURE 6.
Molecular model of the R1-2 protein (methods as in Ref. 27. The tandem repeat is constituted by the two repeats R1 and R2, each of them comprising three helices, HA, HB, and HC for R1 and HA′, HB′, and HC′ for R2. The N and C termini are denoted N and C, respectively The tryptophan residues are denoted as red W, and the L427 site of the mutation is shown in Repeat 1. The region of the mutation is enlarged on the right, showing that the Trp-431 is very near the mutation pointing into the interior of the molecule in the wild-type model. Leucine 427 (dotted gray sphere) points to the interior of the molecule. Therefore, the mutation L427P leads to the proline residue (blue sticks) pointing also to the interior with putative helix breaking; this likely is able to destabilize the folding of these three helices.