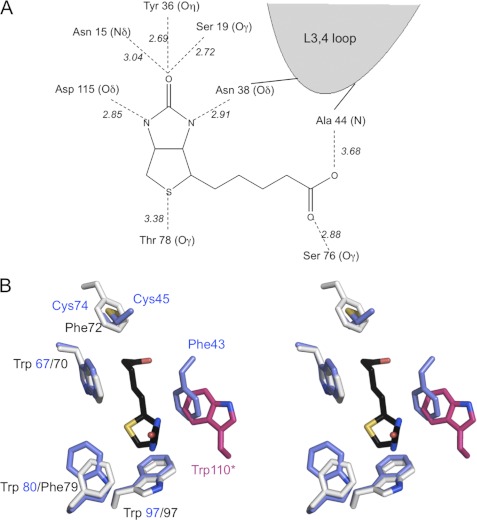
FIGURE 3.
Biotin-binding site. A, schematic representation of the hydrogen-bonding network in the shwanavidin complex with biotin. The biotin ring system forms an identical network of H-bond interactions as in other avidins where five are formed with the uriedo ring and one with the tetrahydrothiophene sulfur. Furthermore, the L3,4 loop contributes a single H-bond interaction with one of the biotin ureido nitrogens. Each of the biotin carboxylate oxygens forms a single H-bond interaction with Ser-76 and main chain nitrogen of Ala-44. B, aromatic residues and the disulfide bridge involved in biotin binding in shwanavidin. Shown is stereo presentation of superimposed residues involved in forming the hydrophobic box accommodating biotin in shwanavidin (light blue) and avidin (light gray). For clarity, the biotin molecule (black) from the shwanavidin complex is shown. The disulfide bridge between the L3,4 and L5,6 loops in shwanavidin accommodates the precise position as the avidin Phe-72 side chain ring. Phe-43 from the L3,4 loop accommodates a highly similar position as Trp-110 of avidin (shown in magenta) contributed by an adjacent monomer. This configuration of aromatic residues in shwanavidin leaves the biotin molecule almost completely unavailable to solvent.