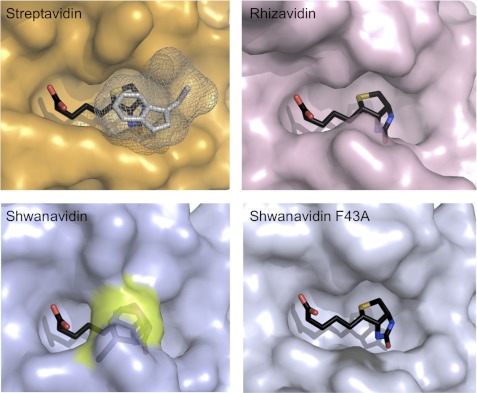
FIGURE 4.
Biotin availability in the binding sites. Surface presentation of the biotin-complexed shwanavidin (light blue), rhizavidin (light pink), and streptavidin (orange) is shown. In streptavidin, the Trp-120 (shown in gray mesh) contributed by a neighboring monomer seals the biotin ligand almost completely. In rhizavidin and shwanavidin an equivalent Trp is lacking due to the dimeric structure. Phe-43 in shwanavidin (shown in the transparent surface and highlighted in yellow) emulates almost exactly the position of the intermonomeric Trp of the tetrameric avidins and caps the biotin molecule in the binding site, rendering the biotin molecule essentially unavailable to solvent. Upon mutating Phe-43 to Ala, the binding site of shwanavidin becomes largely exposed to solvent thus substantiating its low affinity for biotin.