FIGURE 5.
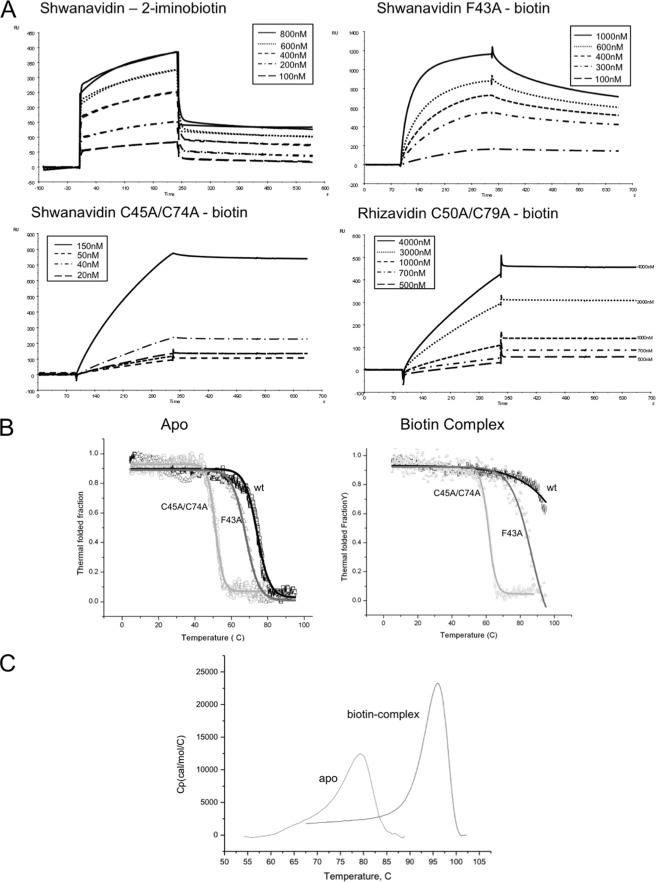
Binding and thermostability properties. A, SPR sensograms showing the association and dissociation of wild-type shwanavidin to 2-iminobiotin. Because the shwanavidin F43A mutant and the disulfide bridge mutants of shwanavidin and rhizaividin did not bind 2-iminobiotin, SPR measurements were conducted using biotin. Protein samples were injected over the chip surface for 240 s at a flow rate of 20 μl/min and then washed with elution buffer without protein for 300 s. Shwanavidin sensograms are similar to those reported previously for rhizavidin (25). The disulfide bridge mutants from shwanavidin and rhizavidin show similar sensograms, although in rhizavidin the Kd is 1 order of magnitude higher than that of shwanavidin. RU, response unit. B, CD thermograms of shwanavidin wild type and mutants (F43A and C45A/C74A). Data were normalized to the thermostable fraction, ranging between 1 (natively folded protein) and 0 (unfolded). Sigmoidal plots of the folded state versus temperature in λ values of 233 and 234 nm for the apo- and biotin-complexed proteins, respectively, clearly show the shift in thermostability upon mutating Phe-43 and the disulfide bridges. C, DSC thermograms of shwanavidin as a function of temperature measured in the absence and presence of biotin in 1 m NaCl, 50 mm NaH2PO4, pH 7. The Tm values of 77.2 and 95.2 °C were obtained at the highest point in the thermograms for the apo- and biotin-complexed shwanavidin, respectively, indicating a higher stability upon biotin binding.