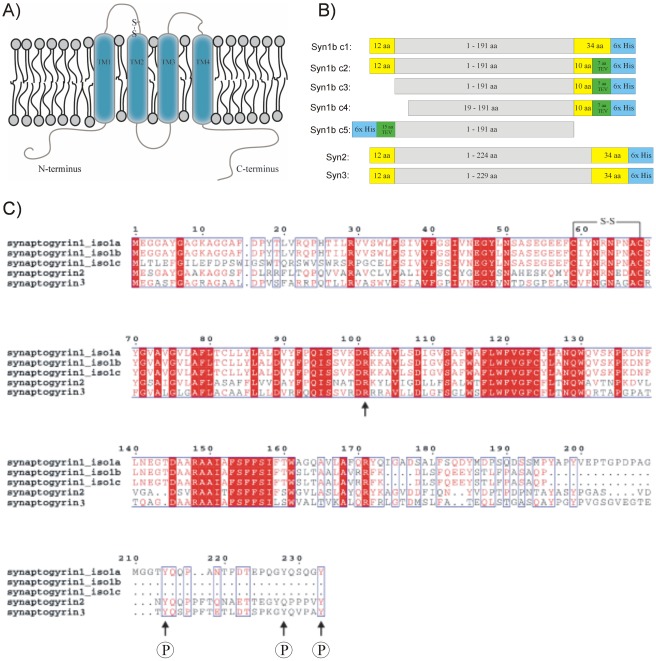
Figure 1. Topology model, construct design and sequence comparison of synaptogyrin.
(A) Topology model and schematic representation of human TVP synaptogyrin in the vesicle membrane. Both, N- and C-termini are predicted to face the cytoplasm. Transmembrane domains are indicated. (B) Synaptogyrin constructs designed for this over-expression study. Additional amino acid residues and affinity tags provided by the vector backbone are color coded. (C) Sequence comparison of the synaptogyrin members 1–3 including isoforms a-c from synaptogyrin 1. Identical and similar residues are color coded in red. The disulfide bridge in vesicular loop 1, a highly conserved arginine in loop 2 and possible phosphorylation sites in the C-terminal part are indicated. Synaptogyrin members show greatest variability in the cytoplasmic C-terminal tail. Synaptogyrin 1 isoforms b and c lack possible phosphorylation sites.