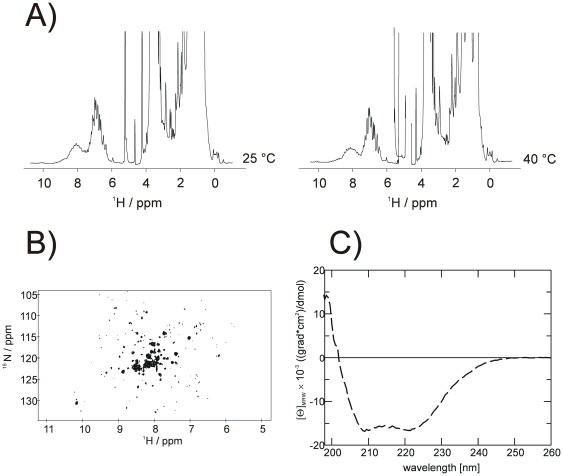
Figure 9. NMR- and CD-spectroscopy of synaptogyrin 1.
(A) One-dimensional proton NMR spectra at two different temperatures as well as the (B) heteronuclear 1H15N TROSY-HSQC spectrum show a well dispersed amid proton signal region (1H = 6 to 9 ppm). The high field shifted aliphatic resonance signals (1H<0 ppm) strongly support a well folded protein state for synaptogyrin 1. (C) Far UV-CD spectrum of synaptogyrin 1 indicates a high degree of alpha helical secondary structure with more than 50 percent of the residues being in an alpha helical conformation.