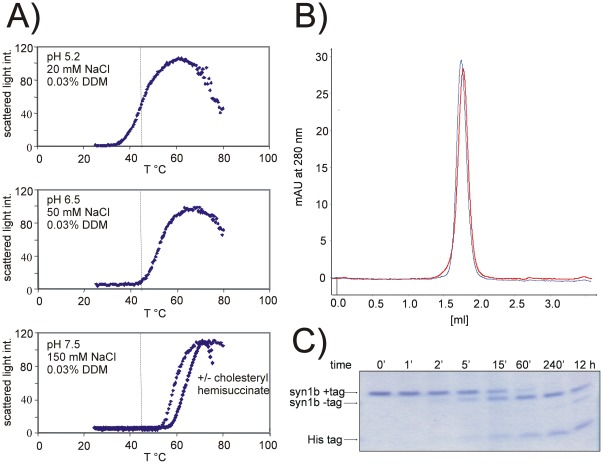
Figure 10. Stability dependence of synaptogyrin 1.
(A) Thermal stability of synaptogyrin 1 was followed via differential static light scattering (DSLS) in a 384 well plate format. The protein is most stable against heat denaturation at neutral pH and physiological concentrations of NaCl. Examples of unfolding curves in three different conditions are shown. The stability is further enhanced in the presence of cholesteryl hemisuccinate. (B) The analytical gel filtration profile is not significantly altered in the presence of 0.03 mg/ml brain lipids and 0.005% cholesteryl hemisuccinate. (−) syn1b in 20 mM sodium phosphate, 150 mM NaCl, 5% glycerol, 0.03% DDM (−) syn1b in 20 mM sodium phosphate, 150 mM NaCl, 5% glycerol, 0.03 mg/ml brain lipids, 0.03% DDM, 0.005% cholesteryl hemisuccinate. (C) Limited proteolysis in the presence of chymotrypsin: Synaptogyrin 1 (construct 5) is stable against proteolytic degradation at a protein to protease ratio of 100∶1. Only the N-terminal His tag is cleaved as verified by mass spectrometry.