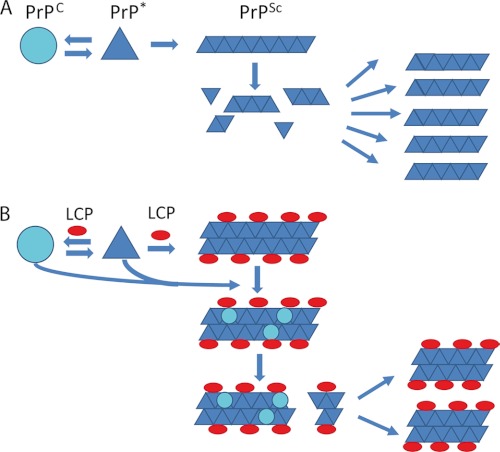
FIGURE 7.
Model for the antiprion activity of the LCPs. A, in the prion model PrPC is in a reversible thermodynamic equilibrium with PrP*, which further aggregates into amyloid fibrils, PrPSc. When the fibrils reach a critical length, the fibril becomes more fragile, and fragmentation occurs. The newly formed ends of the fibril fragments are new nucleation sites for further fibril growth. B, antiprion activity of the LCPs seems to be based on interactions with PrPSc aggregates, possibly increasing their compactness. LCP-coated fibrils further embed pre-existing prions and even PrPC. The higher compactness of the PTAA-treated aggregates ultimately cause less fragmentation into infectious particles necessary for further prion replication.