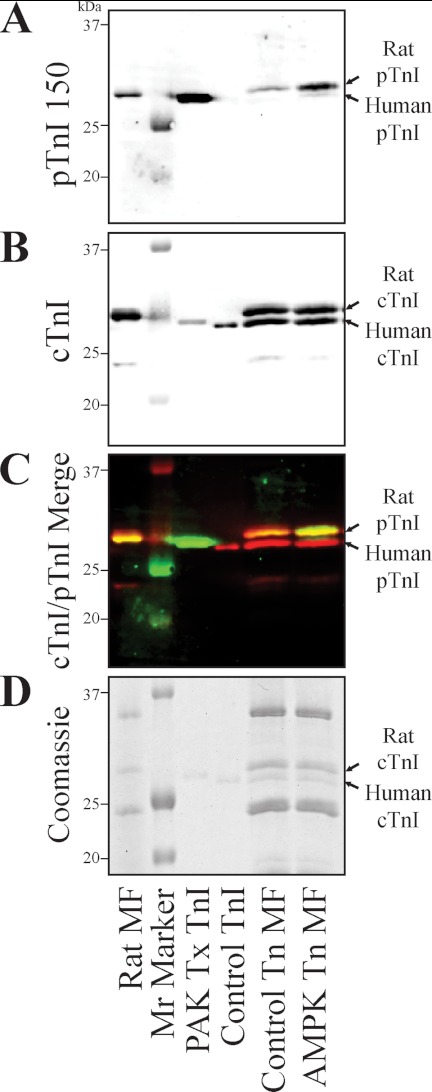
FIGURE 3.
The AMPK holoenzyme phosphorylates cTnI Ser-150 in the cardiac muscle lattice. Rat endogenous cTn in ventricular myofibrils was partially exchanged with recombinant human cTn and incubated in the absence (Control Tn MF) or presence of the AMPK holoenzyme (AMPK Tn MF). A, myofibrils probed by Western blot with the pTnI 150 antibody demonstrates both the remaining endogenous cTnI and the exogenous exchanged human cTnI exhibited increased Ser-150 phosphorylation in the AMPK-treated myofibrils without antibody recognition of non-phosphorylated recombinant cTn (Control Tn). Western also identifies untreated, native rat cardiac myofibrils (Rat MF) as containing endogenous cTnI Ser-150 phosphorylation. B, simultaneous identification of total cTnI by differential detection identified two cTnI bands in exchanged myofibrils of similar size to endogenous rat and exogenous human cTnI at similar loading. C, merge of the pTnI 150 (green) and total cTnI (red) signals demonstrates the pTnI 150 band is identical in size to that of the total cTnI (overlap yellow). D, identical Coomassie-stained gel demonstrates similar loading and integrity of the samples. Mr Marker, molecular mass marker; PAK Tx TnI, Ser-150-phosphorylated human cTnI; Control TnI, unphosphorylated recombinant human cTnI.