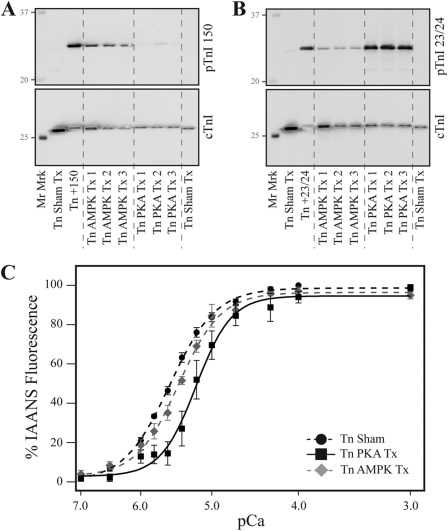
FIGURE 6.
The incorporation of native phosphate at cTnI Ser-150 blunts Ser-23/24-decreased Ca2+ binding to TnC. Recombinant human Tn was treated with PKA or AMPK and reconstituted into thin filaments to determine Ca2+ binding, and cTnI phosphorylation was determined. A, a representative Western blot of filaments with the pTnI 150 antibody demonstrates AMPK incorporation of native phosphate at Ser-150. B, a representative Western blot of filaments with the pTnI 23/24 antibody demonstrates that both AMPK and PKA induce Ser-23/24 phosphorylation at similarly loaded cTnI. C, steady-state Ca2+ binding to TnC demonstrates filaments reconstituted with PKA-treated Tn (Tn PKA Tx; black square, black solid line) exhibited decreased Ca2+ binding affinity to TnC compared with sham-treated fibers (Tn Sham; black circle, black dashed line), whereas Ca2+ binding was not different between filaments containing native phosphate at both the Ser-23/24 and Ser-150 (Tn AMPK Tx; gray diamond, gray dashed line) and sham-treated filaments. Mr Marker, molecular mass marker; Tn +150, cTnI Ser-150 phosphorylated positive control; Tn+23/24, cTnI Ser-23/24-positive control.