FIGURE 2.
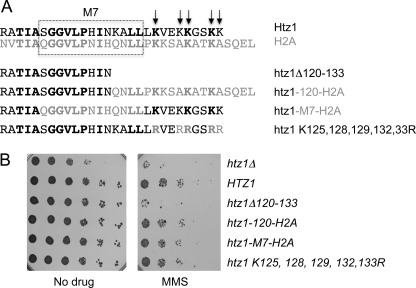
The role of the Htz1 C terminus in MMS resistance can be provided by the H2A C-terminal tail. A, 28 residues at the C terminus of Htz1 (R106 onwards) are aligned with the corresponding region of H2A (top), with residues that are identical in Htz1 and H2A in bold type. The Htz1-H2A hybrid proteins and point mutants are depicted below relative to the Δ120–133 truncation mutant. The M7 region (boxed) or the C-terminal region distal to residue 119 from Htz1 were replaced with the corresponding residues from H2A, which results in 5 or 9 Htz1 residues being substituted with a different amino acid. Arrows indicate the 5 C-terminal lysines that were mutated to arginines. B, serial dilutions of the indicated strains were spotted on plates containing 0.005% MMS or drug-free control plates and incubated for 48–72 H at 30 °C.